Sponsored Content by Cerba ResearchReviewed by Louis CastelMar 16 2023
In this interview, Cerba Research explains how its teams were able to assess clinical response in multiple myeloma (MM) patients that were receiving monoclonal antibodies treatment.
Could you briefly provide our readers with an overview of multiple myeloma (MM)?
In multiple myeloma (MM), a type of bone marrow cancer, malignant plasma cells secrete high levels of monoclonal immunoglobulin protein (M-protein) that can be detected by serum protein electrophoresis (SPEP) or immunofixation electrophoresis (IFE).
International Myeloma Working Group (IMWG) criteria stipulate that patients’ serum samples must present negative results for M-protein by SPEP/IFE to determine a complete response (CR) or stringent CR (sCR).
What role do monoclonal antibodies play, and why are they important?
Monoclonal antibodies have demonstrated therapeutic efficacy in a number of malignancies, but they can impede how IFE data is interpreted. For example, Daratumumab is a CD38 IgG1κ monoclonal antibody (mAb) in clinical development for the treatment of MM5 which has demonstrated clinical responses that intensify over time. This necessitates the evaluation of CR/sCR by SPEP/IFE.
Around half of the number of patients with MM produce an IgG1κ M-protein. In a subset of patients, either daratumumab or the daratumumab–anti-idiotype complex may co-migrate with endogenous M-protein. Steady-state concentrations of daratumumab (dosed at 16 mg/kg weekly, bi-monthly, and then monthly) are readily detectable on the majority of SPEP and IFE assays.
What are the main objectives when assessing clinical response in MM?
The primary goal is to validate and implement a daratumumab interference reflex assay (DIRA) that differentiates M-protein from daratumumab, as assessed by IFE, to establish if additional testing to evaluate CR/sCR is warranted (i.e., bone marrow examination). To do this, we acquire human serum samples from patients with MM (n = 51) from a commercial source or patients treated with daratumumab in clinical trials (n = 33).
How are the clinical samples assessed?
Cerba conducts a series of serum IFE assays using Maxikit Hydragel 9IF Kits (Sebia Electrophoresis, Norcross, GA) in accordance with the manufacturer’s specifications.
Antisera against immunoglobulins gamma (IgG), alpha, and mu heavy chains and free and bound kappa (κ) and lambda light chains are used to determine the monoclonal protein present in each sample.
We then incubate serum samples for baseline and daratumumab-treated patients with or without an anti-idiotype mAb (mouse-anti-huMaxCD38; clone 5-3-9-4) at room temperature for 15 minutes before analyzing with IFE using IgG and Igκ antisera.
To show that the anti-idiotype antibody binds and shifts daratumumab without having an effect on the detection and migration of endogenous M-protein, commercially available serum samples from MM patients (n = 51) were spiked with daratumumab, anti-idiotype, or daratumumab + anti-idiotype (500 and 1000 µg/ml; 1:1 ratio) IgG and Igκ), and were then analyzed by IFE to assess changes in the migration of M-protein.
What are the other key parts of the analysis that must be determined to judge whether the assessment is effective?
There are several factors we have to consider. Lower limit of detection (LOD) is determined by evaluating daratumumab ± anti-idiotype over a clinically relevant dynamic range to establish the lowest concentration detected by ≥1 parameter (daratumumab igg, daratumumab + anti-idiotype complex IgG, daratumumab Igκ, daratumumab + anti-idiotype Igκ for IFE; daratumumab or daratumumab + anti-idiotype by SPEP).
We then ensure reproducibility. We performed three independent runs of 10 samples from daratumumab-treated patients who had achieved PR or better and M-protein ≤5 g/dl. These runs were carried out using DIRA, and the results (DIRA-positive or DIRA-negative) were then assessed for reproducibility. Finally, two independent reviewers check and interpret all results to check concordance.
Once DIRA assessment has been completed, what do you expect to find in the results?
In one specific case, the DIRA template utilized daratumumab ± anti-idiotype as controls for migration of the therapeutic antibody and the daratumumab–anti-idiotype shifted couples.
Baseline and post-treatment serum ± anti-idiotype were then compared to establish whether M-protein remained after shifting daratumumab. DIRA-positive results, in this case, showed M-protein, whereas DIRA-negative results showed only a shift in daratumumab but no remaining M-protein (lanes 8, 12; Figure 4).
We also determined the lower limit of detection (LOD) in MM serum samples, using IFE at 100 µg/ml in 9 of 10 samples by ≥1 parameter and at 200 µg/ml in 10 of 10 samples. In the same samples analyzed by SPEP, either daratumumab and daratumumab plus anti-idiotype complex could be identified at 100 µg/ml in 3 of 10 samples and by 200 µg/ml in 10 of 10 samples.
In 10 of 10 (100%) daratumumab-treated patient samples, results were consistent across all three independent runs, demonstrating a sufficient level of DIRA reproducibility and concordance.
What makes DIRA a good method for assessing clinical response in multiple myeloma?
DIRA enables the identification of clinical responses. In one study, a total of 33 samples from daratumumab-treated patients from several different studies were assessed for a clinical response using DIRA; 13 patients (39%) were DiRA-negative, 10 of whom were confirmed as having achieved CR based on bone marrow and FLC while 20 (61%) were DIRA-positive, meaning they will continue to be monitored.
As mentioned, DIRA is a specific, reproducible method that can confirm the interference of daratumumab on serum IFE at 100 to 200 µg/mL. Moreover, DIRA-negative status signals that additional testing is required to verify CR/sCR and IMWG response criteria may require modification as mAbs receive approval for the treatment of MM.
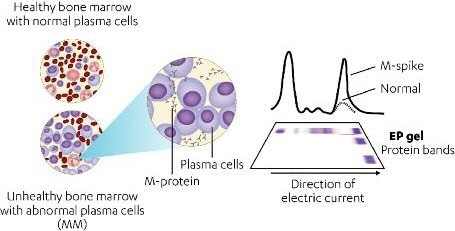
Figure 1. MM cells secrete high levels of M-protein detectable by SPEP. Image Credit: Cerba Research
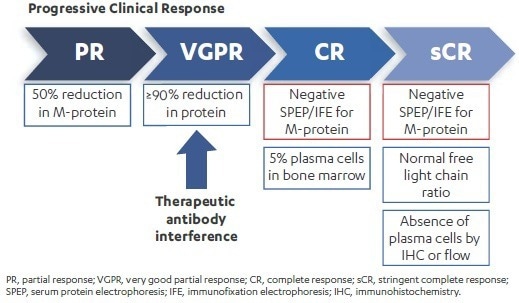
Figure 2. Therapeutic antibodies may interfere with the ability to confirm clinical outcomes deeper than very good partial responses. Image Credit: Cerba Research
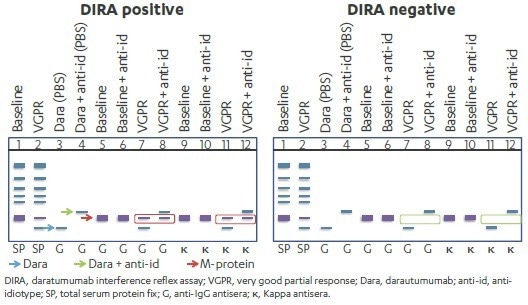
Figure 3. Schematic Presentation of DIRA-positive and DIRA-negative daratumumab-treated Patient Samples. Image Credit: Cerba Research
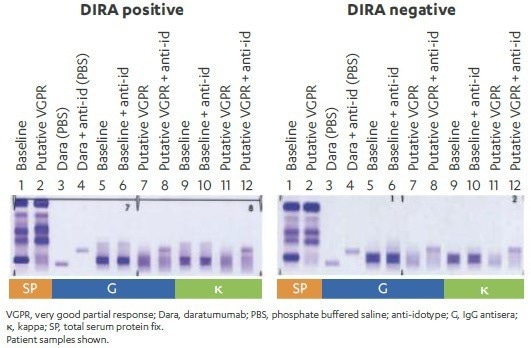
Figure 4. Example of DIRA-positive and DIRA-negative daratumumabtreated patient samples. Image Credit: Cerba Research
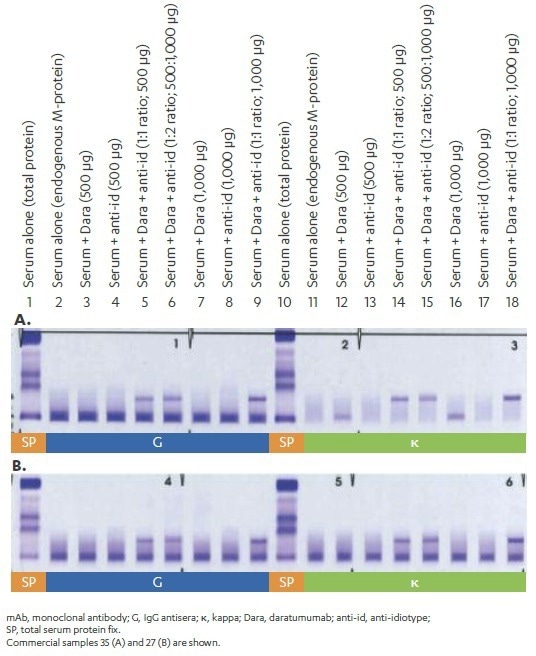
Figure 5. Specificity of anti-idiotype MAb. Image Credit: Cerba Research
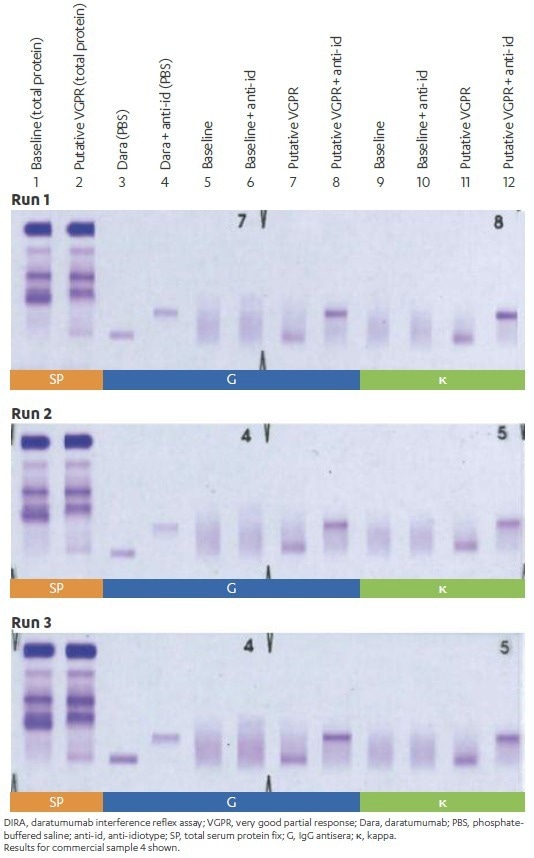
Figure 6. Reproducibility of DIRA results between independent experiments. Image Credit: Cerba Research
Table 1. Concordance of Reviewer Assessments Between Experiments. Source: Cerba Research
| Reviewer 1 |
Lane |
Run 1 |
Run 2 |
Run 3 |
| Migration of Dara + anti-id in control |
4 vs. 3 |
y |
y |
y |
| Migration of endogenous M-protein at baseline |
6 and 10 |
n |
n |
n |
| Migration of Dara in VgPR due to the disappearance of Dara (DD) or the appearance of Dara + anti-id complex (AC) |
8 vs. 7
and
12 vs. 11 |
y
DD + AC |
y
DD + AC |
y
DD + AC |
| Presence of M-protein after migration of Dara |
8 and 12 |
n |
n |
n |
| M-protein (M) or Dara (D) |
D |
D |
D |
| Conclusion |
|
negative |
negative |
negative |
| Reviewer 2 |
Lane |
Run 1 |
Run 2 |
Run 3 |
| Migration of Dara + anti-id in control |
4 vs 3 |
y |
y |
y |
| Migration of endogenous M-protein at baseline |
6 and 10 |
n |
n |
n |
| Migration of Dara in VgPR due to the disappearance of Dara (DD) or the appearance of Dara + anti-id complex (AC)? |
8 vs 7
and
12 vs 11 |
y
DD + AC |
y
DD + AC |
y
DD + AC |
| Presence of M-protein after migration of Dara? |
8 and 12 |
n |
n |
n |
| M-protein (M) or Dara (D)? |
D |
D |
D |
| Conclusion |
|
negative |
negative |
negative |
Dara, daratumumab; anti-id, anti-idiotype; y, yes; n, no; VgPR, very good partial response.
About Cerba Research
For over 35 years, Cerba Research has been setting the industry standard for exemplary clinical trial conduct. Today, across five continents, with a focus on precision medicine, we are changing the paradigm of the central lab’s role in complex clinical research.
From protocol inception through development and to market, our passionate experts deliver the highest quality specialized and personalized laboratory and diagnostic solutions. Partner with us for the most efficient strategy to actualize your biotech and pharmaceutical products sooner and improve the lives of patients worldwide.