The fast and simple tracking of primary and metastatic cancer development research in live animals is enabled within vivo imaging using fluorescent proteins. Discovered decades ago in the jellyfish Aequorea victoria as a primary feature of bioluminescence1, a green fluorescent protein (GFP) is a commonly utilized fluorescent dye for in vivo imaging.
Upon determining its novel utility for fluorescence labeling in other living organisms, the development of new molecules has expanded the range of available fluorescent proteins to encompass almost the complete visible spectrum from 350 nm (UV) to 750 nm (Infrared).
The enhanced properties and unique characteristics of fluorescent proteins have since permitted the non-invasive visualization of dynamic processes and structural organization in living cells and organisms by in vivo imaging2. This technology enables subjects to be monitored longitudinally over the course of a study to supply real-time information on internal changes.
Application of in vivo imaging can be seen over a multitude of disciplines from medicine and drug discovery to cell biology and physiology. Yet, there is usually extensive training and specific experience involved working with live animals when preparing in vivo samples with fluorescent-labeled proteins.
In addition to complicated sample preparation procedures, imaging the fluorescent proteins within small animals requires specialized instrumentation consisting of an excitation light source, a high-performance camera, emission filters, and associated software.
These requirements for acquiring images and preparing samples largely limits opportunities for entry-level researchers or students to participate in research involving in vivo applications.
An easy-to-use entry-level imager, the UVP GelSolo, is an ideal option for multi-user laboratories, school laboratories, and practical training where users may not have access to relevant samples or experience with in vivo imaging.
In order to practice in vivo imaging methods and get a taste of how in vivo imaging works in a laboratory setting, researchers are quickly able to work with the UVP GelSolo and a silicone mouse sample.
This article will show how in vivo fluorescent imaging of a typical mouse model employed in research labs is carried out, substituting a fluorescent silicone mouse.
Equipped with a 5.0-megapixel camera, UV transilluminator, 8-48mm f/1.2 manual zoom lens, white and blue overhead LED light sources, the UVP GelSolo will be employed to image the silicone mouse.
With a peak wavelength at 450nm, the Blue LED light source is a commonly employed excitation source for green and red fluorescent protein labels utilized for in vivo imaging.
Students at non-research institutions who are interested in learning in vivo imaging but might not have access to animal models can use the UVP GelSolo to practice the in vivo imaging method.
School laboratories can utilize these instruments for teaching purposes and can use this article to supplement STEM training, particularly students aiming for advanced degrees and research careers.
Materials and methods
The UVP GelSolo was employed to image a fluorescent silicone mouse embedded with both red and green round plastic fluorescent rods. The fluorescent rods were selected because red fluorescent protein (RFP) and green fluorescent protein (GFP) are frequently used for in vivo imaging.
Figure 1 shows their spectral profiles, blue LED Light, having a wavelength of 445 nm to 470 nm and a peak wavelength near 456 nm, which can excite these fluorescent proteins which lie in the GFP excitation spectrum.
The emission filter for GFP has been selected as 510 nm to 523 nm with a peak wavelength of 513 nm. The emission filter designed for RFP has a wavelength range of 575 nm to 640 nm and a peak wavelength of 605 nm.
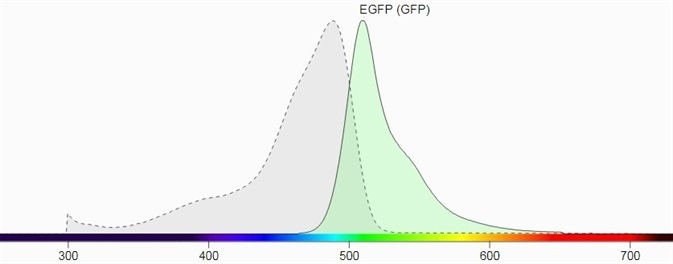
Figure 1. a) Excitation and emission spectrum of GFP (Source: Biotium). Image Credit: Analytik Jena US
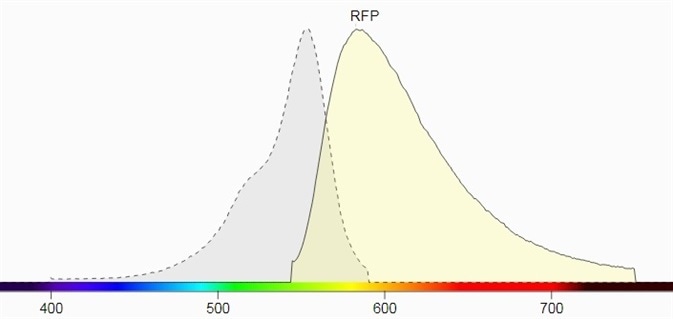
Figure 1. b) Excitation and emission spectrum of RFP (Source: Biotium).
Silicone mouse creation
The mouse model which was utilized for this experiment is created from colored silicone rubber. In order to mimic the look of the true nude mouse model commonly used in fluorescent in vivo imaging white and red and acrylic paints were applied.
Two pieces of 1 cm red round plastic fluorescent rods were inserted into the head of the silicone mouse and two pieces of 1 cm green-yellow round plastic fluorescent rods were inserted into the back of the mouse before the silicone model was completely solidified to visually mimic tumors. The silicone rubber mouse then set for four hours and was subsequently imaged.
Silicone mouse imaging
The UVP GelSolo imager was used to image the silicone mouse. Selections for exposure time, light sources, and emission filter are shown in Table 1.
Table 1. Capture Parameters for GFP and RFP Signal.
| Capture Parameters |
White |
GFP |
RFP |
| Exposure Time |
150 ms |
3 seconds |
2 seconds |
| Excitation Light |
White LED |
Blue LED |
Blue LED |
| Emission Filter |
Clear |
510 nm-523 nm, Peak 513 nm |
575 nm-640 nm, Peak 605 nm |
Image acquisition step-by-step procedure
- Turn on the Epi White light on the system.
- Place the silicone mouse in the darkroom.
- Open the filter slot located on top of the system and place the 513 nm and 605 nm emission filters into the filter slots. Leave one filter slot empty for capturing white light images.
- Open the VisionWorks software on the desktop and begin Live View.
- Adjust the manual lens until the previewed image is sharp and clear.
- Click Capture to acquire a white light image.
- Turn off Epi White light and turn on Epi Blue light.
- Slide the filter tray until the 513 nm filter is in place.
- Start Live view and capture the green fluorescent image.
- Switch the filter to 605 nm and capture the red fluorescent image.
- The Capture parameters for both GFP and RFP signals are shown in Table 1 and captured images can be seen in Figure 2.
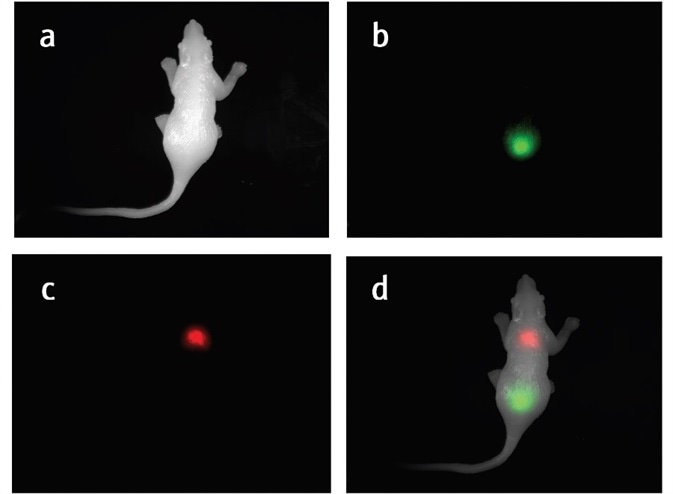
Figure 2. a) White image of the silicone mouse. b) GFP signal in the silicone mouse. c) RFP signal in the silicone mouse. d) Composited white channel, GFP channel, and RFP channel to help visualize the GFP and RFP signal.
Results
The silicone mouse and the individual GFP and RFP signals are clearly detected with the UVP GelSolo imaging system, as demonstrated in Figure 2a-c. Figure 2d shows the location of each signal in a composite image.
The results exhibit the distinct imaging of fluorescent signals within an organism in an easily reproducible and non-invasive manner. In addition to the in vivo image acquisition method, researchers and students will also be able to learn post-processing for captured in vivo images.
The emission filter blocks unwanted wavelengths and permits only the chosen wavelengths to pass through, as seen in Figure 2b and c. The RFP emission filter permits signals with 575 nm to 640 nm to pass through and the GFP emission filter permits fluorescent signals with wavelengths of 510 nm to 523 nm to pass through.
These images confirm that the emission filter blocks unwanted signals efficiently and provide a clean image. However, it is difficult to establish where the signal is located. In this instance, the compositing and pseudocolor functions in the software become useful.
Usually, depending on the spectrum of the fluorescent protein as shown in Figure 2b and c, the fluorescent signal will be pseudo-colored. Compositing the colored fluorescent images with a white light image helps to locate the fluorescent signals on the animal.
The intensity of the signal can be analyzed using the Area Density feature in the software for a more in-depth analysis. As an easy-to-use system, the UVP GelSolo is designed for imaging and analysis without the requirement for any additional training.
The straightforward features and user-friendly automation make the UVP GelSolo an ideal entry-level imager for multi-user laboratories, school laboratories, and practical training.
References and Further Reading
- Day, Richard N and Michael W Davidson. “The fluorescent protein palette: tools for cellular imaging” Chemical Society reviews vol. 38,10 (2009): 2887-921.
- Dmitriy M. Chudakov, Mikhail V. Matz, Sergey Lukyanov, and Konstantin A. Lukyanov. “Fluorescent Proteins and Their Applications in Imaging Living Cells and Tissues” Physiological Reviews 2010 90:3, 1103-1163
About Analytik Jena US
Analytik Jena is a provider of instruments and products in the areas of analytical measuring technology and life science. Its portfolio includes the most modern analytical technology and complete systems for bioanalytical applications in the life science area.
Comprehensive laboratory software management and information systems (LIMS), service offerings, as well as device-specific consumables and disposables, such as reagents or plastic articles, complete the Group’s extensive range of products.
About life science
The Life Science product area demonstrates the biotechnological competence of Analytik Jena AG. We provide a wide product spectrum for automated total, as well as individual solutions for molecular diagnostics. Our products are focused to offer you a quality and the reproducibility of your laboratory results.
This will surely ease your daily work and speed up your work processes in a certain way. All together we support you through the complete process of the lab work. Besides we offer customized solutions and are able to adapt our products to your needs. Automated high-throughput screening systems for the pharmaceutical sector are also part of this segment’s extensive portfolio.
About analytical instrumentation
Analytik Jena has a long tradition in developing high-performance precision analytical systems which dates back to the inventions made by Ernst Abbe and Carl Zeiss. We have grown to become one of the most innovative manufacturers of analytical measuring technology worldwide.
Our business unit Analytical Instrumentation offers excellent competencies in the fields of optical spectroscopy, sum parameters and elemental analysis. Being proud of our core competency we grant all our customers a long-term warranty of 10 years for our high-performance optics.
About lab automation
With more than 25 years of market experience, Analytik Jena with its CyBio® Product Line is a leading provider for high quality liquid handling and automation technologies. In the pharmaceutical and life science industries, our products enjoy the highest reputation for precision, reliability, robustness and simplicity.
Moreover, the Automation Team designs, produces and installs fully automated systems tailored to our clients' application, throughput and capacity requirements. From stand-alone CyBio® Well up to fully customized robotic systems we handle your compounds, biomolecules and cells with great care.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.