Clinical trials of new drugs to treat the most aggressive form of prostate cancer are expected to be underway in Brisbane within three years, thanks to Movember Revolutionary Team Award grant that is fast-tracking global research into the disease.
QUT Professor Colleen Nelson, who is head of the Australian Prostate Cancer Research Centre-Queensland (APCRC-Q), will lead a team of international researchers who have been awarded $4.25 million from the Movember Foundation to develop better treatments through greater collaboration.
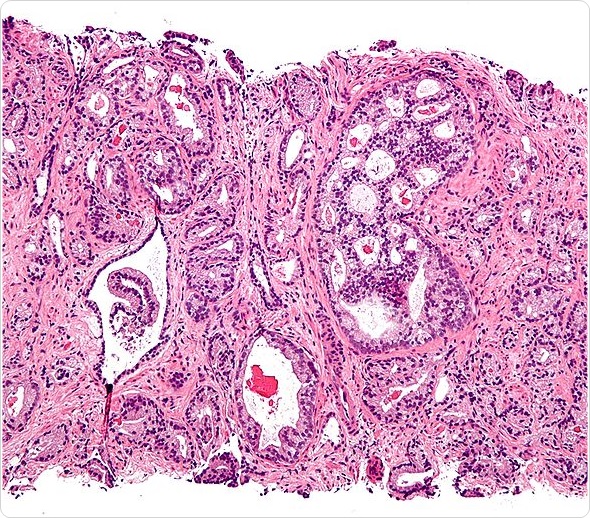
Micrograph showing prostatic acinar adenocarcinoma (the most common form of prostate cancer) Gleason pattern 4. H&E stain. Prostate currettings.
Professor Nelson said further leveraging and in-kind support would see the funding almost double.
"Prostate cancer is a global problem and the philosophy behind the Movember Foundation funding is that lead researchers and their teams collaborate across borders and disciplines to develop a far better understanding of all aspects of this disease and to achieve results more quickly," she said.
Professor Nelson will lead an international team of 39 researchers, 15 of whom are based at APCRC-Q with collaborations involving researchers in Vancouver, Dublin, Sydney, Melbourne and Adelaide.
Professor Nelson said the team would develop new drugs and treatments for prostate cancer and gain a better understanding of why some forms of prostate cancer became resistant to current treatments, particularly Androgen Targeted Therapies (ATT).
"More than 25 per cent of men who are diagnosed with prostate cancer will go on to have advanced stage of the disease and ATTs are the most common treatments," she said.
"While ATTs treat the cancer - however the cancer ultimately becomes resistant to them - and they have side effects which lead to patients developing conditions such as Type 2 diabetes and fragile bones.
"ATTs activate and reorder many of a patient's metabolic pathways and we aim to develop strategies to target the activated genes to alleviate these negative effects.
"As well, a range of blockbuster drugs targeting androgen action has come onto the market in recent years and by sharing information we will develop a super-high resolution of the characterization of the impact each of these drugs has on prostate cancer, how they are similar, where they are distinctive, and this will enable us to develop a world first high-resolution data repository of the differential responses of ATTs."
Professor Nelson said researchers would then better understand how the drugs could be used and in what combinations and sequences to have greatest impact.
Based at the Translational Research Institute (TRI) in the Princess Alexandra Hospital campus, Professor Nelson said the APCRC-Q was perfectly situated to translate the team's research into new drugs that would be used in clinical trials within TRI's Clinical Research Facility.
She said ultimately collaboration would result in a change to the way in which prostate cancer was managed and treated by bringing to the forefront a greater understanding of the disease and the impact current treatments had on metabolic dysfunction and the impact this had on patients' wellbeing and quality of life.