Cell Signaling Technology (CST), a life science biotechnology company and leading provider of antibodies, kits, and services, and Amoy Diagnostics Co., Ltd. (AmoyDx), a China-based innovative molecular diagnostics company in the field of precision oncology, announced today the expansion of their ongoing partnership for companion diagnostic (CDx) development in China. As the use of precision oncology therapeutics continues to advance in China, the partnership will provide AmoyDx with access to additional, highly validated CST® antibodies against critical CDx targets that will support the development of diagnostic assays used to identify patients for targeted therapeutics.
 Immunohistochemical analysis of paraffin-embedded human lung carcinoma using PD-L1 (E1L3N®) XP® Rabbit mAb #13684.
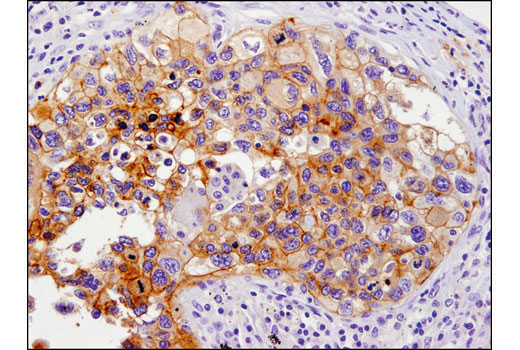
Immunohistochemical analysis of paraffin-embedded human lung carcinoma using PD-L1 (E1L3N®) XP® Rabbit mAb #13684.
“By working with partners like AmoyDx, CST is helping to enable the translation of preclinical research into cutting-edge therapeutics and diagnostic solutions that can save the lives of cancer patients,” said Roberto Polakiewicz, Chief Scientific Officer at CST. “AmoyDx’s industry-leading molecular diagnostics and clinical services are at the forefront of innovation, and our ongoing partnership will help move precision oncology forward by providing the best-in-class antibodies needed for developing specific and sensitive CDx assays.”
In March 2022, AmoyDx received approval for the company’s PD-L1 Expression Detection Kit (IHC), which leverages the CST PD-L1 antibody validated for immunohistochemistry (IHC) and is used to guide the first-line treatment of Pembrolizumab in non-small cell lung cancer patients. With the expanded agreement in place, the companies will work together to identify additional antibody products against crucial targets that best support AmoyDx’s growing diagnostic portfolio, with a focus on enabling precision medicine for oncology therapeutics.
“We are happy to extend our collaboration with CST to create additional companion diagnostics that can help identify patients that would benefit from targeted therapeutics,” said Jiemin Luo, PhD, General Manager of AmoyDx. “AmoyDx is committed to being a leading global supplier of quality diagnostic products and services for personalized healthcare. Through this strengthened relationship and our combined expertise in oncology therapeutics pathways, we hope to bring more precision diagnostics to the clinic to support the needs of patients in China.”
CST is a leading provider of highly specific and sensitive antibody products, including an extensive oncology research portfolio. In 2022, the most recent year for which data is available, CST was named the antibody Supplier Succeeding in Cancer Research by CiteAb, and over one-third of the products in CiteAb’s Top 100 Cited Antibodies of 2022 were CST products, more than any other antibody supplier.
About Cell Signaling Technology
Cell Signaling Technology (CST) is a different kind of life science company—one founded, owned, and run by active research scientists, with the highest standards of product and service quality, technological innovation, and scientific rigor. Founded in 1999 and headquartered in Danvers, Massachusetts, USA, CST employs over 600 people worldwide. We consistently provide fellow scientists around the globe with best-in-class products and services to fuel their quests for discovery. CST is a company of caring people driven by a devotion to facilitating good science—a company committed to doing the right thing for our customers, our communities, and our planet. cellsignal.com
About Amoy Diagnostics Co., Ltd. (AmoyDx, SZSE: 300685)
Amoy Diagnostics Co., Ltd. is a pioneer and global leading company in the field of molecular diagnostics for precision oncology, focusing on companion diagnostics product development and commercialization. AmoyDx has a portfolio with more than twenty products approved by China’s NMPA, the EU regulatory authorities, Japan’s MHLW, South Korea’s MFDS, etc. Patients in more than 60 countries are benefiting from AmoyDx products. With multiple technological platforms and full capability for companion diagnostics product development and commercialization, AmoyDx has become an important diagnostics partner for many pharmaceutical companies around the globe. For more information, please visit www.amoydiagnostics.com.