Dynamic light scattering (DLS) is a technique used to determine particle size distributions of bioformulations. Traditionally, DLS measurements have been used for studying dilute solutions, but contemporary DLS instruments can help in measuring diffusion coefficient of samples at high concentrations and high turbidity. In the bioformulation market, these systems are used widely. However, there are four basic issues which should be considered when analyzing the effect of sample concentration on DLS-based analysis of bioformulations. These issues include restricted diffusion, multiple scattering, electrostatic repulsion and reversible self-association. It is possible to detect each of these effects while conducting a concentration-dependent DLS experiment.
Concentration Effects - Multiple Scattering
As and when the concentration of a sample is increased, an interaction occurs between a scattered photon and another macromolecule, resulting in a rescattering effect, as shown in Figure 1. This effect is referred to as multiple scattering.
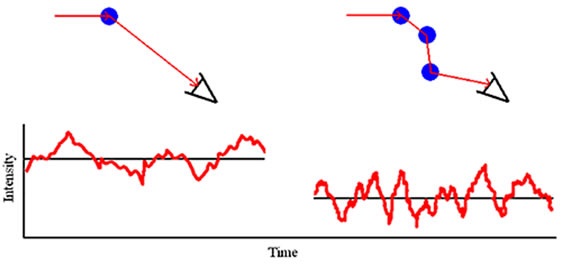
Figure 1. Multiple scattering schematic
As the concentration of the sample is increased the following occurs:
- The intercept of the correlation curve will reduce because of the reduction in the quantified scattering intensity
- The correlation curve will decay quickly owing to the arbitrariness of multiple scattering events
- The scattering intensity will reduce because of the reduction in the light scattered toward the detector with each rescattering event
For samples having multiple scattering, increased sample concentration will lead to an increase in the distribution width, polydispersity and a decrease in the mean size. Figure 2 illustrates the effect of multiple scattering on DLS results for a PEGylated biotherapeutic.
Under extreme dilute conditions, the low scattering intensity not only results in reduced correlogram intercept, but also leads to increased polydispersity of the distribution.
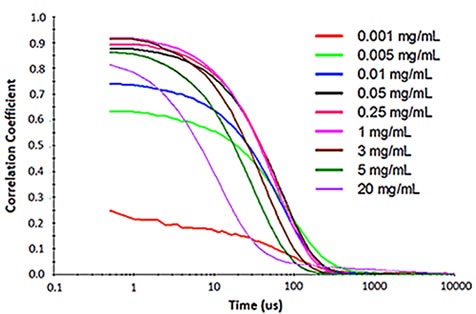
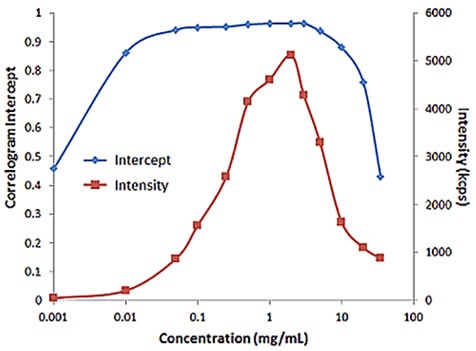
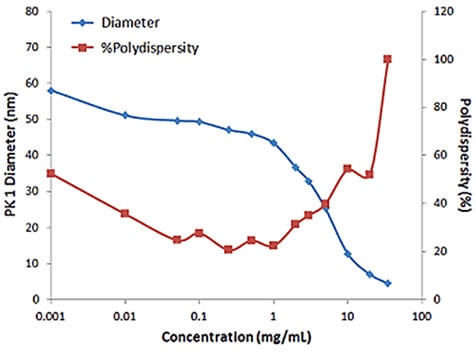
Figure 2. Influence of multiple scattering on DLS results for a PEGylated biotherapeutic.
The sample concentration at which multiple scattering takes place depends on the particle size being determined. However there is no apparent delineation between samples displaying multiple scattering and samples displaying single scattering.
According to Pine and Weitz (Dynamic Light Scattering: The Method and Some Applications), the photon mean free path governs the diffusive limit of multiple scattering. By applying Mie theory, the mean free path can be measured when sample concentration, laser wavelength, refractive index of dispersant and particles, and particle diameter are known. The measurement of the mean free path is then used to delineate the size- and concentration-dependent minimum particle separation distance for samples exhibiting single scattering. Figure 3 depicts the size dependence of the highest sample concentration for single scattering DLS through a 633nm laser for aqueous protein systems.
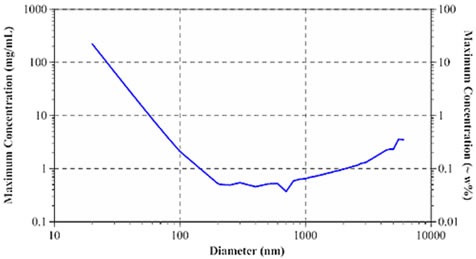
Figure 3. Size dependence of maximum sample concentration for single scattering DLS.
The Zetasizer Nano is an advanced backscatter system that can offset multiple scattering effects by calculating near the cell wall. This helps in reducing the distance between the detector and the scattering center. Backscatter systems can significantly boost the maximum concentration for single scattering. However, backscatter technology is suitable for opaque or opalescent samples, but not so for water-clear biosamples.
Restricted Diffusion
Diffusion or Brownian motion is the outcome of thermally-driven collisions of dispersant molecules with the macromolecule. Traditionally, DLS measurements were preformed under dilute solution conditions. The dispersant viscosity was utilized to justify the viscous drag of the surrounding medium. As the concentration of the sample increases, a viscous component is imparted to the surrounding medium by other macromolecules, as shown in Figure 4. This concentration effect is referred to as restricted diffusion. The effects of restricted diffusion effects can be detected during the course of a DLS dilution experiment.
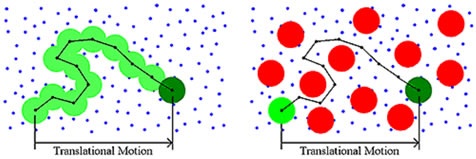
Figure 4. Schematic depicting concentration-dependent restricted diffusion effects.
As and when the concentration of the sample is increased, the DLS size distribution will move toward larger particle sizes when the dispersant viscosity is utilized. Since restricted diffusion has an additive or determinant effect, no change will be seen in the width or modality of the distribution.
Instead of using the dispersant viscosity, bulk viscosity can be used to correct the restricted diffusion effects in the Stokes-Einstein equation. Figure 5 displays the bulk viscosity corrections for an emulsion having restricted diffusion effects.
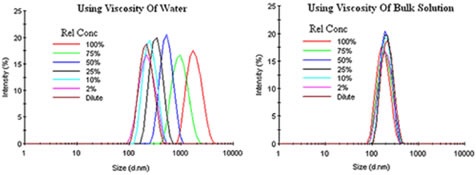
Figure 5. Bulk viscosity corrections for an emulsion exhibiting restricted diffusion effects.
Virial Effects
The second virial coefficient or B22 is a thermodynamic parameter, which represents the interaction potential between adjacent proteins in a bioformulation. Although the interaction potential comprises all types of interactions, electrostatic interactions dominate in protein formulations. Therefore, they can be easily identified when analyzing the concentration effects on DLS measurements. Positive virial coefficient values denote repulsive interactions, while negative virial coefficient values indicate attractive interactions.
Attractive - Reversible Self-Association
Proteins having negative virial coefficient values will display reversible self-association. This kind of concentration effect is called attractive virial effect, wherein the macromolecule favors self-association over solvation. Although DLS is not capable of resolving oligomeric species, the R6 dependence of the scattering makes this technique highly sensitive to even small changes in the oligomeric distribution. Therefore, attractive virial effects can be detected during the course of a DLS dilution experiment. Figure 6 displays the DLS dilution results obtained for an antibody sample having attractive virial effects.
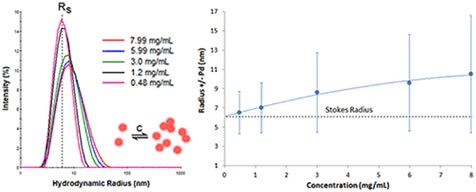
Figure 6. DLS dilution results for an antibody formulation exhibiting reversible self-association or attractive virial effects.
Repulsive - Electrostatic Colloidal Stability
Proteins having positive virial coefficient values will display electrostatic repulsion. Since this repulsion reduces aggregation, positive or repulsive virial coefficient effects indicate colloidal stability. During the course of a DLS dilution analysis, new DLS users will find it difficult to detect repulsive virial effects. It is important to remember that DLS determines particle motion.
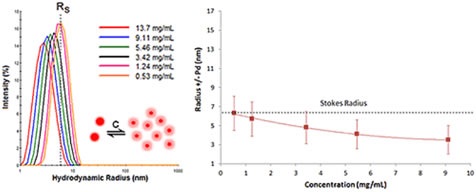
Figure 7. DLS dilution results for an antibody formulation exhibiting electrostatic repulsive virial effects.
Figure 7 displays the DLS dilution results obtained for an antibody sample with repulsive virial effects.
DLS Interaction Parameter (kD)
The DLS interaction parameter (kD) is used to measure particle interactions. As mentioned before, experimental correlations between the DLS interaction parameter and API solubility, formulation viscosity, and protein aggregation rates have been reported, and the parameter is being utilized for screening biotherapeutic formulations and candidates to indicate stability at high concentration. The predictive value of kD is the result of the effect of the second virial coefficient (B22) on kD, as shown in the expression below:
D=D0 (1+kDC)
kD=2B22MW-(kf+2υ)
Where B22 is the second virial coefficient, D0 is the self-diffusion coefficient at the limit of zero concentration (C). MW is the molecular weight, υ is the partial specific volume, and kf is the first order concentration coefficient of the friction coefficient.
Figure 8 clearly shows the effect of B22 on kD, which reveals a comparison of the Dynamic Debye plots for antibody formulations shown in Figures 6 and 7, respectively.
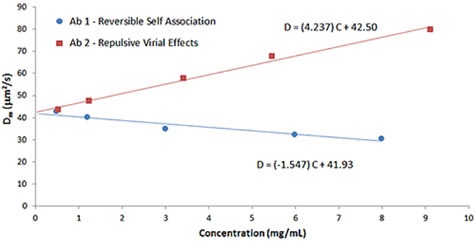
Figure 8. Comparison of Dynamic Debye plots for antibody formulations exhibiting reversible self-association and repulsive virial effects.
As mentioned before, positive B22 values indicate samples that favor salvation over self-association, while negative B22 values indicate samples that prefer self- association. As shown in Figure 8, the antibody formulation having reversible self-association exhibits a negative kD value, while the formulation having stabilizing repulsive virial effects exhibits a positive kD value that are in line with B22 predictions.
Conclusion
This article covers the influence of particle interactions and concentration effects on DLS results and provides a roadmap for detecting and differentiating each type of concentration effect. When studying the effects of sample concentration on DLS analysis of protein therapeutic formulations, issues such as restricted diffusion, electrostatic repulsion, multiple scattering, and reversible self-association should be taken into account.
About Malvern Panalytical

Malvern Panalytical provides the materials and biophysical characterization technology and expertise that enable scientists and engineers to understand and control the properties of dispersed systems.
These systems range from proteins and polymers in solution, particle and nanoparticle suspensions and emulsions, through to sprays and aerosols, industrial bulk powders and high concentration slurries.
Used at all stages of research, development and manufacturing, Malvern Panalytical’s materials characterization instruments provide critical information that helps accelerate research and product development, enhance and maintain product quality and optimize process efficiency.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.