During the development of biopharmaceuticals, decision has to be made at the outset whether a biopharmaceutical will be delivered in powder or liquid form. To that end, both powder and liquid formulations have their own advantages and disadvantages. End user convenience is also another factor that has to be taken into account.
Proteins unfold or denature upon heating or when denaturing chemicals are mixed into the solution. Unfold proteins are more sensitive to deamidation, oxidation, and proteolysis processes, which can promote inactivation. Such proteins can also aggregate, which in turn can result in loss of stability and protein breakdown. Before the formulation development, it is important to characterize protein, which may involve the determination of its amino acid composition, molecular weight, 3D structure, solubility, and other parameters. Such data is useful for making optimal protein formulation.
DSC and Formulation Development
Differential scanning calorimetry or DSC is a microcalorimetry method utilized to differentiate a biomolecule’s stability in its native form. The measurement of the thermal transition midpoint (Tm) indicates stability When the Tm is higher, the biomolecule will be more stable.
A DSC instrument features a sample cell comprising biomolecule and buffer as well as a buffer-filled reference cell. When temperature is increased, the temperature variation between the reference cells and sample is monitored by the instrument. The variation in heat uptake between the cells determines the excess heat capacity, as shown in Figure 1.
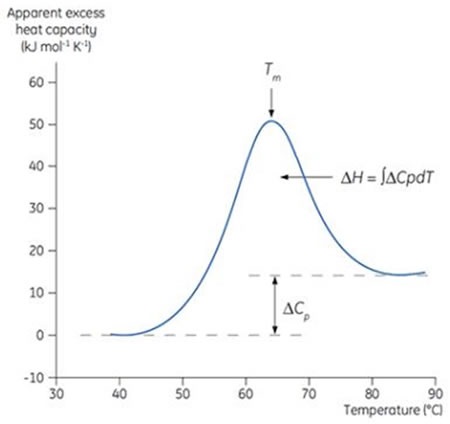
Figure 1: Typical DSC thermogram. This DSC scan was carried out on a dilute protein solution, where the protein undergoes a transition from a compact, native state at low temperature to an unfolded, denatured state at high temperature. The apparent excess heat capacity of the protein was measured, based on the difference in the heat capacity of the protein in buffer, and buffer alone.
The Tm for the enthalpy change occurs when the protein changes from folded (native) to denatured form. At the Tm, half of the protein is in denatured state and other half is in the native state, presuming a two-state transition (Figure 1).
Tm indicates thermostability. When Tm is higher, the protein is less likely to unfold and denature at a lower temperature. By interrogating different additives and conditions, DSC has the ability to determine the highest Tm formulations that will match with the optimal formulations for stability.
The change from native to denatured protein is often endothermic. During the conformational transition, the change in enthalpy (ΔH) is determined by integrating the area under the transition, as shown in Figure 1. When compared to the native protein, the heat capacity (Cp) of the unfolded o denatured protein is higher which results in a positive ΔCp for thermal denaturation.
The Malvern Panalytical MicroCal VP-DSC and Malvern Panalytical MicroCal™ VP-Capillary DSC (Figure 2) systems are utilized for analysis of biopolymers in solution. The latter system has been developed for Tm screening of different formulations at high sample throughput with a quick scan rate. Table 1 compares the features of the Malvern Panalytical MicroCal VP-DSC and Malvern Panalytical MicroCal VP-Capillary DSC systems.

Figure 2. Malvern Panalytical MicroCal VP-Capillary DSC system.
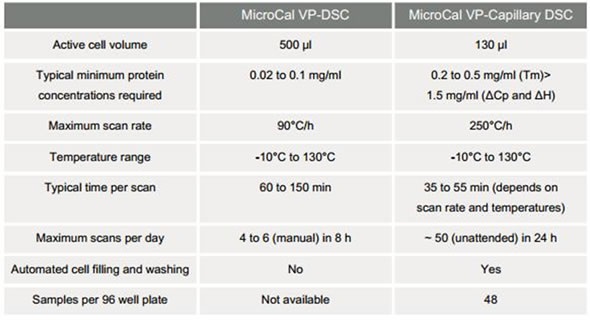
Table 1. Comparison of Malvern Panalytical MicroCal VP-DSC and Malvern Panalytical MicroCal VP-Capillary DSC systems
Liquid Formulation Strategies
In any process of formulation development, it is critical to find out which solution conditions provide optimal stabilization of folded protein. Conditions resulting in the highest Tm sustain the protein in its folded state for the longest period of time at lower temperatures. Through the DSC technique, different buffer and pH conditions are screened first, with subsequent screening of excipients and preservatives.
Optimization of pH and Buffer Conditions
The Tm of protein CD40L was plotted versus pH (Figure 3). The CD40L aggregation was also ascertained following incubation at 37°C for 1 week. The Tm optimum matched with the pH conditions where there was minimum aggregation. This correlation between Tm, pH, and aggregation was also observed with macrophage colony stimulating factor.
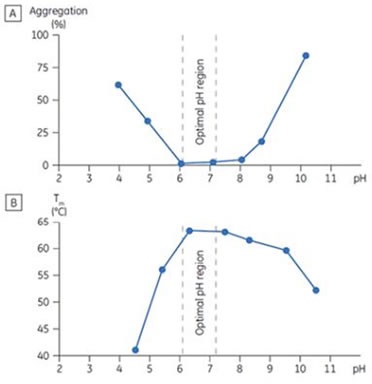
Figure 3. Stability behavior of CD40 ligand (CD40L) correlating aggregation response (A) as determined by size exclusion chromatography (SEC), and (B) the Tm determined by DSC as a function of pH. The bracketed area represents the optimal pH range where Tm is maximized and aggregation is minimized.
The Tm changes of proteins can be easily measured with the DSC technique. Figure 4 depicts the Tm changes of chymotrypsinogen upon increased pH. These changes were determined with the Malvern Panalytical MicroCal VP-Capillary DSC and indicate improved stability of the folded form of chymotrypsinogen at higher pH.
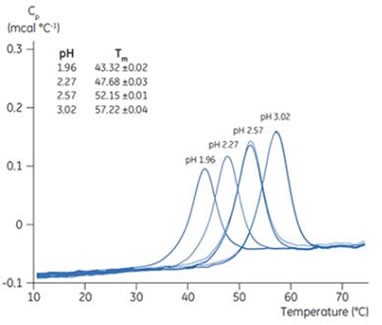
Figure 4. Tm shift of chymotrypsinogen with pH. Chymotrypsinogen solutions (pH 1.96, 2.27, 2.57, and 3.02) were prepared and added to a 96-well plate. Five samples were used for each pH. Matched reference buffers were also placed in the 96 well plate. DSC scans were performed with Malvern Panalytical MicroCal VP-Capillary DSC. The DSC data shown here are after buffer-buffer reference scan subtraction. The inset has the Tm data for each pH, and standard deviation.
Excipients
Excipients such as sugars, antioxidants, amino acids, etc. enhance the stability of proteins. After determining optimum buffer and pH conditions, different excipients are mixed to the protein solution. If the Tm is increased by an excipient, the protein in native form is more stable with the added excipient. During the development of interleukin-1 receptor, IL-1R, excipient screening was employed. In order to screen for excipients, the transition with the Tm at lower temperature, i.e., 48°C was chosen. The idea was to search for excipients that can increase the Tm of the low temperature transition, and thus indicate a positive transition in the stability of native protein (Table 2).
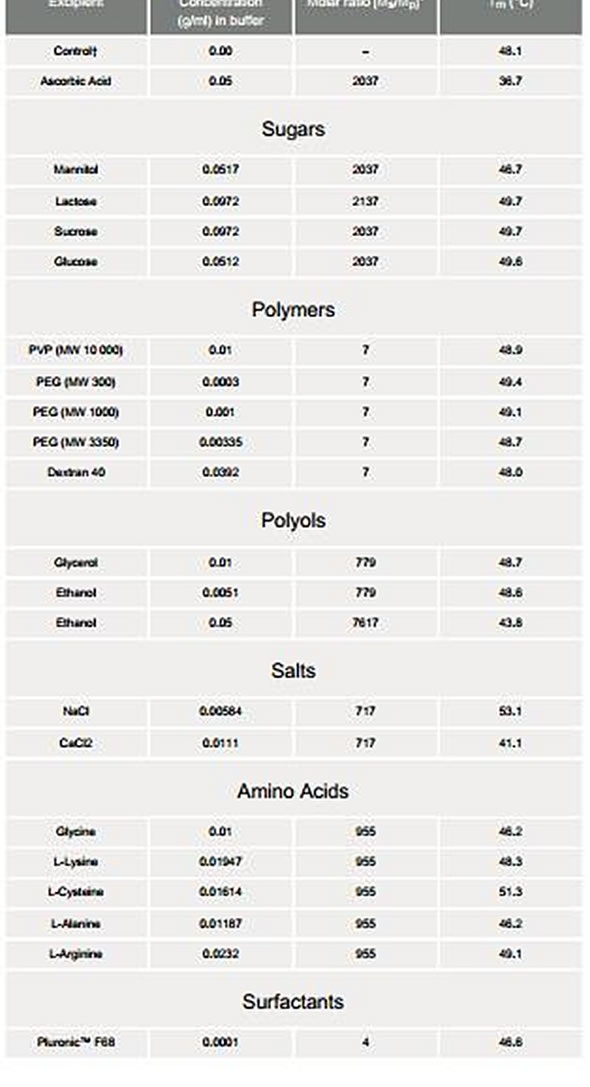
Table 2. Screening of excipients added to interleukin-1 receptor
Ionic Strength
The buffer’s ionic strength was modified to determine whether Tm can be increased with the addition of salt. In case of IL-1R, 100 mM NaCl was added which had the highest stabilizing effect, at ionic strength moving the Tm from 48°C to 53°C. This stabilizing influence indicated a direct interaction between the protein’s charged groups and salt ions. Even when NaCl is 1500 mM, the Tm of IL-1R increases with increasing salt which is well over the concentration required to saturate all the charged sites, as shown in Figure 5. These results imply that water structure is affected by salt ions. Changes in water structure as well as charge-charge interactions add stability to the IL-1R structure.
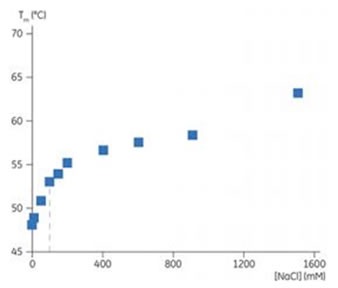
Figure 5. Plot showing Tm of IL-1R with addition of NaCl. The 100 mM concentration is shown by the dashed line.
Preservatives
Preservatives are used to prevent microbial growth, particularly when a drug is delivered in a multidose format. These preservatives, however, may have a destabilizing influence on the protein. The phenol, meta-cresol, and benzyl alcohol are preservatives that destabilized IL-1R, depending on the change of temperature transitions to lower temperatures, as shown in Table 3.
The highest Tm was generated by phenol, meta-cresol, and benzyl alcohol, respectively. When the Tm was higher, less aggregation was seen after 7 and 60 days.
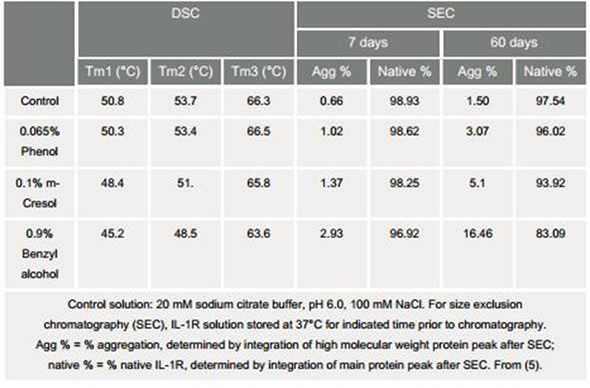
Table 3. Effects of preservatives on IL-1R: Comparison of Tm and size exclusion Chromatography
Based on Tm screening, the optimum formulation candidates were then assessed by accelerated stability analyses. The protein made in different formulations was stored at 37°C. Size exclusion chromatography was used to determine the amount of aggregation at regular intervals throughout the accelerated stability analysis and the protein was examined by means of SDS-PAGE to screen for proteolysis.
Real-time stability studies were then performed on the formulation candidates to ascertain the shelf life of the protein. In order to make sure that the protein remains viable and active, analytical tests and bioassays were carried out during the study.
Conclusion
DSC serves as an excellent screening tool and is used to evaluate the stability of proteins during formulation development. With the help of the Malvern Panalytical MicroCal VPCapillary DSC system, faster screening and high throughput can be realized. This system not only helps in determining the Tm of proteins in different excipients and pHs, but also aids in observing the changes in the Tm linked to the SEC data. These factors reinforce the application of the DSC technique as a stability screening tool. Moreover, with the use of DSC, significant amount of time and money can be saved in formulation development. Fully automated solutions allow for better efficiency and productivity in this vital aspect of drug development.
About Malvern Panalytical

Malvern Panalytical provides the materials and biophysical characterization technology and expertise that enable scientists and engineers to understand and control the properties of dispersed systems.
These systems range from proteins and polymers in solution, particle and nanoparticle suspensions and emulsions, through to sprays and aerosols, industrial bulk powders and high concentration slurries.
Used at all stages of research, development and manufacturing, Malvern Panalytical’s materials characterization instruments provide critical information that helps accelerate research and product development, enhance and maintain product quality and optimize process efficiency.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.