During biopharmaceutical drug development, differential scanning calorimetry (DSC) is used to evaluate protein stability. The data provided by this established biophysical tool is employed to advance the most 'developable' and stable proteins into the pipeline, and improve the formulation and process conditions to sustain protein stability during manufacturing and storage of drugs.
The thermal transition midpoint (TM) is determined by DSC. For a protein that reversibly denatures, TM is the temperature where half of the protein is in its folded or native conformation, and half is in its unfolded or denatured conformation.
TM is a good indicator of thermal stability – if the TM is higher, the protein is more thermally stable. During DSC, if a protein is irreversibly denatured, the TM offers key insights about its thermal stability. Antibodies are multi-domain proteins that often have more than a single unfolding domain, and multiple TMs are detected by DSC.
In fact, in a recent survey, biopharmaceutical scientists rated DSC as a 'very useful' to 'extremely useful' biophysical tool for product characterization, formulation development, candidate selection, biosimilarity, and comparability [1].
Introduced in 2002, the MicroCal VP-Capillary DSC system was the first automated DSC developed for biopharmaceutical applications. This system is designed to perform TM screening during pre-formulation and formulation development of biopharmaceuticals.
The data contained from this DSC system are also employed to assess protein stability during protein engineering, candidate selection, process development, biosimilar development, higher order structure (HOS) characterization during the course of comparability studies, and manufacturing support.
Biopharmaceutical facilities, CRDOs, and CROs across the globe are using MicroCal VP-Capillary DSC systems, whose usage has been mentioned in various book chapters, journal articles, and reviews.
New MicroCal PEAQ-DSC systems
Malvern Panalytical has introduced the new MicroCal PEAQ-DSC Automated system in 2017. The system has new improvements and features when compared to the MicroCal VP-Capillary DSC. These upgrades enhance productivity during the discovery and development of biopharmaceuticals, producing high-quality, reliable, and reproducible DSC data.
- New PEAQ-Compliance: These tools help employ MicroCal PEAQ-DSC Automated system data in a regulated environment (refer #1 below)
- New PEAQ-Performance: These tools support performance checks on standards as well as optimized DSC cell cleaning protocols to enhance data quality and provide confidence for producing reproducible DSC thermograms (refer #2 below)
- New PEAQ-Smart experimental design software: This intuitive control software enables quick and flexible experimental setup (refer #3 below)
- New PEAQ-Smart data analysis software: This is an automated and objective DSC data analysis that also includes automated TM detection. A new report designer is included in the PEAQ-Smart (refer #4 below)
- New PEAQ-Compare: This software simultaneously studies DSC thermograms and compares to a reference thermogram. It is designed for biosimilarity and comparability studies (refer #5 below)
- New Autosampler: with reduced amounts of sample requirements (refer #6 below) and more robust components, needs less frequent replacements of needle seals (refer #7 below)
- New controller: with connectivity to communicate with network and send emails (refer #7 below).
- New high-concentration protein sample protocol: to be available soon (refer # 8 below)
1. DSC data analysis in the regulated environment: PEAQ-compliance
For novel biopharmaceutical drugs and biosimilars, DSC data are increasingly being included in regulatory submission packages. Documentation contains DSC stability data for proposed manufacturing changes to recognized processes, to show that the changes do not have any effect on the end drug product. In addition, there is a growing interest to include DSC as a Quality Control assay during the manufacture of biopharmaceuticals.
In the case of a regulated environment, the instrument user has to show data integrity. In US FDA’s document, “Data integrity and Compliance with cGMP: Guidance to Industry (April 2016)”[2], guidelines have been provided.
One of the requirements is that creation and modification of all the records are auditable and controlled. The FDA 21 CFR Part 11 or EU GMP Annex 11 covers this requirement and was written to allow pharmaceutical companies to submit electronic data. Origin 7 software is used by MicroCal VP-Capillary DSC systems, but it does not support 21 CFR Part 11 compliance requirements.
PEAQ-Compliance software has tools that support FDA 21 CFR Part 11 compliance. The software is available as an optional feature for MicroCal PEAQ-DSC systems:
- Electronic records from MicroCal PEAQ-DSC contain audit trail and event log such as creation and modification of records
- Electronic records from MicroCal PEAQ-DSC contain metadata such as date and time, user IDs, method parameters, and instrument type
- No modification is made to original data files; all metadata relating to original electronic records are retained by copies
- Malvern Panalytical Access Configurator (MAC) is used to configure and control all access to electronic records
- MicroCal PEAQ-DSC user log-in credentials and PEAQ-Compliance software provide electronic signatures to electronic records through Windows security system; enables security access levels to be configured according to organizational needs
Performance Qualification (PQ) of the instrument is another component of 21 CFR Part 11 compliance that includes regular performance checks. A new tool called PEAQ-Performance is included in the MicroCal PEAQ-DSC systems, and this enables performing and assessing performance check data (refer #2).
2. Increase DSC productivity and provide confidence in results: PEAQ-Performance
Performance checks
Regular "performance check" experiments are standard operating procedures (SOPs) for MicroCal VP-Capillary DSC systems, where a well-defined "standard" with an established DSC thermogram is used. These experiments are used to check the DSC instrument, which is key to producing high-quality, reproducible, and reliable DSC data.
Regular performance checks reduce the requirement for multiple replicate experiments and at the same time increases user’s confidence in the DSC data. These performance checks are also a component of Performance Qualification (PQ), needed for data analysis in a regulated environment (refer #1 above).
During DSC experiments, Microcal VP-Capillary DSC users were not automatically informed about the failed performance checks, and experiments had to be again performed once the instrument issues were dealt with.
The MicroCal PEAQ-DSC Automated systems are provided with the new tool named PEAQ-Performance. Performance check experiments on standards are included in the sample set at preferred intervals. The standard DSC data is automatically evaluated by PEAQ-Performance software.
In case the performance check fails, i.e., it does not meet the defined tolerances of DSC parameters, then the instrument operator can program the instrument to either stop further experiments or clean the DSC cells, or continue the remaining programmed experiments, or be alerted through email. As a last option, the operator can decide the subsequent steps after receiving the notification.
DSC cell cleaning
High-quality DSC data can be generated using clean DSC cells. In a normal SOP with MicroCal VP-Capillary DSC automated systems, the DSC cells have to be rinsed with Contrad 70 (or Decon 90) solution after a sample is scanned, and one or two buffer-buffer reference scans are performed prior to the next sample scan. The buffer-buffer scans act as an extra instrument performance check to ensure that the DSC cells are indeed clean and that the instrument is working seamlessly.
To clean the MicroCal VP-Capillary DSC cells rigorously, detergent solution is added in the 96-well plate, and a cleaning 'scan' is programmed, followed by a single buffer-buffer scan. These SOPs is a time-consuming process and use up the space in the sample trays, thereby reducing productivity and sample throughput.
Three pre-programmed DSC cell cleaning protocols are included in PEAQ-Performance that are optimized for the MicroCal PEAQ-DSC Automated system:
- detergent wash (followed by water rinse)
- water rinse
- detergent scan (fill cells with detergent, execute scan, clean with water)
The novel autosampler with MicroCal PEAQ-DSC systems has enhanced sample handling, leading to effective and faster cell cleaning and minimizing carry-over and contamination. If an optimized DSC cell cleaning protocol is used, productivity can be considerably increased by carrying out three or more DSC experiments with high-quality data, and that too with no buffer-buffer scans in between.
Figure 1 illustrates thermograms for 16 DSC scans of 8 different batches of ribonuclease A, without any buffer-buffer scans between the protein scans.
DSC system warm-up
In order to ensure optimal performance, MicroCal VP-Capillary systems need multiple 'warm-up' scans. A warm-up feature is included in the MicroCal PEAQ-DSC systems that detects when the instrument is ready and automatically begins the experiments. As a result, the warm-up scan time is reduced, providing confidence in data quality.
Fast scan rates
With regard to TM screening experiments, the MicroCal PEAQ-DSC systems use fast scan rates (up to 240 °C/hour) to create high-quality thermograms. Another way to save time and increase productivity is to use fast scan rates.
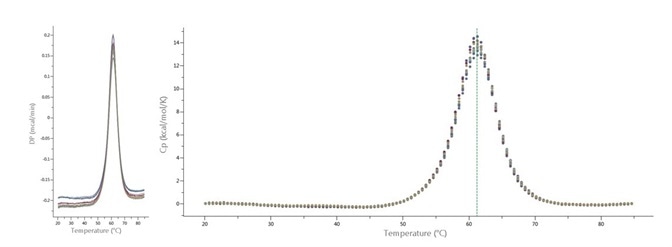
Figure 1. Sixteen thermograms of ribonuclease A (60 μM, or 0.88 mg/mL) analyzed with MicroCal PEAQ-DSC Automated system. DSC temperature range was 15-85 °C, at 200 °C/h scan rate. DSC cells were cleaned with 'detergent wash' after each sample. Buffer-buffer scans were performed before first protein scan and after final protein scan. Left: Raw data as displayed by PEAQ-Smart software. Data are two replicate scans of 8 different batches of protein. Right: after data analysis and fitting with PEAQ-Smart software. Green line represents TM determined by PEAQ-Smart.
Figure 1 depicts 16 thermograms of ribonuclease A conducted on a MicroCal PEAQ-DSC Automated system at a scan rate of 200 °C/hour.
The experiments performed using MicroCal VP-Capillary DSC automated system at a scan rate of 200 °C/hour would take roughly 33 hours to conclude a total of 40 DSC scans, including 16 buffer-buffer scans (prior to each sample scan), 4 warm-up scans, 2 detergent scans, and 1 performance check scan (with matched buffer-buffer scan).
When the MicroCal PEAQ-DSC Automated system was used, it took 20 hours for the 16 sample thermograms to complete a total of 24 scans including two warm-up scans, two detergent scans, two buffer-buffer scans, and a performance check protein scan.
In this case, about 13 hours are saved by using the MicroCal PEAQ-DSC Automated system, which help complete an additional 15 scans for each day. This translates to an increased productivity of about 75%.
3. Increase productivity with fast, flexible experimental sample and scan set-up: PEAQ-Smart
PEAQ-Smart software is integrated in the MicroCal PEAQ-DSC Automated systems for programming DSC experiments. Sample templates in the software enable common configuration elements to be re-used rapidly. In addition, the template setup makes it easy to move and duplicate experiments.
Standard experiments like sample, buffer-buffer, and performance check are color coded, and if required, all DSC experiments can be unique in cell cleaning method, temperature range, scan rate, and other parameters. The methods can be developed on the user’s computer and the same can be transferred to the instrument; they can also be saved for later use.
In studies focused on reversibility of unfolding, the MicroCal PEAQ-DSC Automated system can be programmed for multiple rescans of the same sample. This can be achieved using different temperature ranges for the rescans.
It must be remembered that the MicroCal VP-Capillary DSC Automated system control software can merely program rescans with the same settings as the initial scan. The MicroCal PEAQ-DSC Automated system can also be programmed to perform downscans (cooling scans) following an upscan (heating), but this option was not provided in the VP-Capillary DSC Automated system.
4. Increase productivity with automated, objective data analysis: PEAQ-Smart
It can be difficult to analyze multiple data files from MicroCal VP-Capillary DSC, and often multiple mouse-clicks had to be made to process the data. With the help of the PEAQ-Smart data analysis software, multiple data sets can be processed quickly and simultaneously, including creation of integration baselines, subtraction of buffer-buffer baseline, and fitting data to measure TM and the overall enthalpy. A report designer is also included in PEAQ-Smart for data presentation.
Typically, monoclonal antibodies have two to four unfolding transitions in DSC, with each transition having its own TM. A 'hit box' in the MicroCal VP-Capillary DSC data analysis software identifies TMs from DSC thermograms; it can 'miss' transitions with shallower peaks in the thermogram, meaning the user have to process each thermogram manually.
The MicroCal PEAQ-DSC systems feature a new algorithm called PEAQ-Finder tool, which automatically establishes more than one TM from DSC thermograms, including peaks that are missed by Origin software. A comparison of the hit box for Origin and TM finding for PEAQ-Finder is shown in Figure 2.
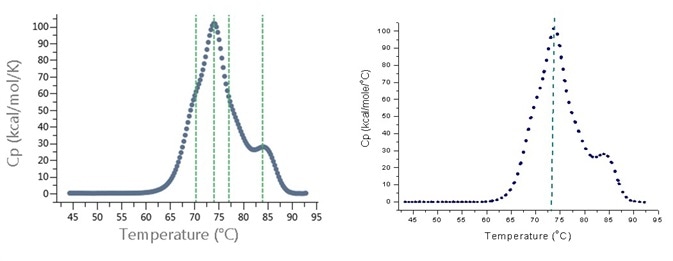
Figure 2. TM identification in DSC thermogram of monoclonal antibody sample. Left: After analysis with PEAQ-Smart and PEAQ-Finder software tool from MicroCal PEAQ-DSC system. The software identified four TMs, indicated by green dashed lines. Right: After analysis with Origin DSC data analysis and hit box from MicroCal VP-Capillary DSC. The software identified only one TM, indicated by green dashed line.
5. Objective data analysis for comparability and biosimilarity studies: PEAQ-compare
DSC is often integrated in HOS and biophysical assays for biopharmaceutical comparability in order to show that the protein product developed is 'highly similar' in comparison to a reference lot of the same protein. After making considerable changes to the manufacturing process, a comparability exercise is performed to assess how these changes affect the key quality attributes of the protein product.
As a biopharmaceutical product, biosimilar is highly similar to an earlier approved biological product that is generally made by another company (the innovator, parental, or reference biopharmaceutical). In comparison to the parental drug, a biosimilar exhibits a highly similar DSC profile 'fingerprint'. This is demonstrated by using DSC as a HOS biophysical assay.
MicroCal PEAQ-DSC systems feature a new software tool named PEAQ-Compare, which carries out evaluation and data analysis on a set of DSC thermograms, and affords a quantitative, objective similarity comparison to a reference DSC thermogram. The PEAQ-Compare proves handy for biosimilarity and comparability researches.
6. Reduced sample requirements
Using the new autosampler equipped with the MicroCal PEAQ-DSC Automated system, the user can set 325 μL of protein sample in the tray well (per scan), but MicroCal VP-Capillary DSC Automated systems will require 370 to 400 μL of the same protein sample.
To obtain high-quality DSC data, standard SOPs for the MicroCal PEAQ-DSC system will need protein concentrations of 0.5 to 1 mg/mL. For numerous protein samples, high-quality DSC thermograms with 0.05 mg/mL protein can be obtained (Figure 3), and for TM screening, lower concentrations of protein can also be used.
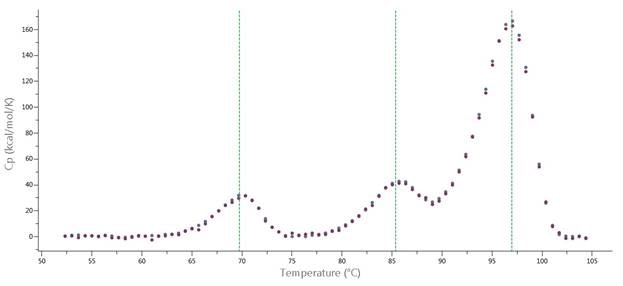
Figure 3. Data from MicroCal PEAQ-DSC Automated system, at 240 °C/hour scan rate. Thermograms of NISTmAb at 0.167 mg/mL (red) and 0.0555 mg/mL (blue) after data analysis with PEAQ-Smart software. Green lines represent TMs determined by PEAQ-Smart.
7. DSC system is critical to workflow
If a facility largely depends on DSC for its workflow, then instrument down-time can cost both time and money and slow down the institute’s research and drug development activities. MicroCal PEAQ-DSC Automated systems now come with a new controller, a new and more robust autosampler design, and more robust firmware.
A computer in the controller networks to the institution’s servers, and the installed software is ready for emails. The autosampler includes a number of components (including the needle seal) that are more robust and durable when compared to MicroCal VPCapillary DSC automation.
The latest MicroCal PEAQ-DSC Automated system includes several features to boost productivity, as mentioned above. Software licensing enables data analysis offline, even under 21 CFR Part 11 compliance, and the provided software also enables saving data directly to a corporate network.
8. DSC for analysis of high-protein concentrations (available by December 2017)
There are many biopharmaceutical protein formulations that are greater than 100 mg/mL. If MicroCal VP-Capillary DSC systems are used, high protein concentrations would need to be used which can cause protein gelling following thermal denaturation.
This causes contamination that cannot be removed easily and cleaning the DSC cells will lead to instrument downtime. Therefore, DSC analysis is now being performed at concentrations that are much below the high-concentration formulations.
A method has been developed by Malvern Panalytical that enables the use of MicroCal PEAQ-DSC systems with high-concentration of protein samples. The high-concentration procedure and the required software and equipment will be launched by the end of 2017. Users can add these components to their existing MicroCal PEAQ-DSC Automated systems.
NOTE: Malvern Panalytical also provides the MicroCal PEAQ-DSC system without an autosampler.
Specifications and new features of MicroCal PEAQ-DSC Automated system, compared to MicroCal VP-Capillary DSC:
|
Specification/feature
|
MicroCal PEAQ-DSC Automated
|
MicroCal VP-Capillary
DSC (with autosampler)
|
|
Tools to support 21 CFR Part 11 compliance
|
PEAQ-Compliance (optional feature)
|
Not available
|
|
Performance check: Programmed standard experiments auto analyzed
|
Included in PEAQ-Performance. If performance check fails, user has option to program cell cleaning, stop further DSC scans, continue experiments, or notify user by email
|
Not available
|
|
Multiple standardized cell cleaning methods
|
Included in PEAQ-Performance. Three pre-programmed cell cleaning options
|
Two cell cleaning options
|
|
Automated warm-up
|
Included in PEAQ-Performance
|
Not available
|
|
Data analysis
|
PEAQ-Smart: Fast, intuitive, automated, data analysis with PEAQ-DSC software
|
Origin software. Limited functionality
|
|
TM determination for DSC transitions
|
PEAQ-Smart and PEAQ-Finder, automated TM determination of multiple thermograms
|
Origin software hit box
|
|
Automated subtraction of matched buffer-buffer scan
|
Included in PEAQ-Smart. More options for choosing buffer-buffer scan for subtraction compared to Origin software
|
Included in Origin software, limited options to choose buffer-buffer scan
|
|
Automated integration baseline generation and determination of ΔH
|
Included in PEAQ-Smart, improved algorithms and to select pre- and post transitions; progress, spline, and linear integration baselines
|
Included in Origin software, limited options compared to PEAQ-Smart; one integration baseline option
|
|
Email sent to user
|
Available
|
Not available
|
|
Networking software
|
Yes: PEAQ-DSC software is ready to network
|
Limited with Origin software
|
|
Control software for easy, intuitive experimental setup
|
Included in PEAQ-Smart. Includes templates. More options and features in experimental design compared to VP-Viewer
|
Included in VP Viewer control software, limited options in experimental design
|
|
Video-enabled workflows
|
Included with PEAQ-Smart
|
Not available
|
|
Compare DSC thermograms to reference sample DSC scans
|
Included with PEAQ-Compare
|
Not available
|
|
Data presentation
|
Included with PEAQ-Smart, more options
|
Limited options
|
|
Report designer
|
Included with PEAQ-Smart
|
Not available
|
|
Sorting/'binning' results after data analysis
|
Included with PEAQ-Smart
|
Limited options with Origin software
|
|
Recommended sample volume in tray wells
|
325 μL
|
370-400 μL
|
|
Fully integrated automated liquid handling system
|
New Autosampler. Improvements include: Injection needle with bottom-detector (permits reduced sample volume in well plates); faster wash station optimized DSC cell cleaning; new needle seal (longer lifetime, reduced carry-over)
|
Complete precision XYZ robotic arm. Three thermostatically controlled drawers, each holding two 96-well microtiter plates. 10-port injection valve. Integrated wash station for rapid, high volume cleaning. Injection syringe.
|
|
Controller
|
Windows 10 OS. All PEAQ-DSC software pre-loaded.
|
Windows 7 OS. All Origin software pre-loaded.
|
|
Downscanning for reversibility studies
|
PEAQ-Smart includes option to schedule downscanning after upscanning experiment
|
Not available
|
|
Program unique scan conditions in set of experiments
|
Included in PEAQ-Smart. More options and features in experimental design compared to VP-Viewer
|
Included in VP Viewer control software, limited options in experimental design
|
|
Rescan samples for reversibility studies
|
Included in PEAQ-Smart. Rescans can be different scan conditions compared to 1st scan
|
Included in VP- Viewer. Rescans have to be identical scan conditions compared to 1st scan
|
|
Use high concentration protein samples
|
Method available by end of 2017
|
Not available
|
|
Pressure perturbation calorimetry (PPC)
|
Not available for MicroCal PEAQ-DSC Automated systems (Available as an option for non-automated MicroCal PEAQ-DSC systems)
|
Not available
|
Specifications and features which are the same for MicroCal PEAQ-DSC Automated system and MicroCal VP-Capillary DSC (with autosampler)
|
DSC cell composition
|
Tantalum-61
|
|
DSC cell design
|
Fixed-capillary cell design
|
|
DSC cell active cell volume
|
130 μL
|
|
Models available for DSC data analysis
|
Includes: two-state unfolding; non-two-state unfolding; KD of ligand binding by TM shift.
|
|
Operating temperature range for DSC scans
|
- 10 to + 130 °C (Using upscan mode at 60 °C/hour. When using upscan mode at 200 °C/ hour, the upper temperature limit is 115 °C) (NOTE: Operation between -10 to +2 °C on request upon instrument order)
|
|
Minimum response time
|
5 Seconds*
|
|
Noise
|
0.05 μCal/°C*
|
|
Baseline repeatability
|
1.5 μCal/°C*
|
|
Measurement repeatability
|
<0.2 μCal/ °C *(rescans of stable buffer)
|
|
Measurement reproducibility (intra-instrument)
|
<0.08 °C St. Dev. TM and < 2% RSD on ΔH (Ribonuclease A)
|
|
System reproducibility (inter-instrument)
|
System reproducibility <0.1 °C St. Dev. TM and < 5% RSD on ΔH (Ribonuclease A on MicroCal PEAQ-DSC alpha instruments)
|
|
Multiple feedback modes
|
Three (passive, high gain, low gain)
|
|
Maximum scan rate for upscanning
|
240 °C per hour
|
|
Power feedback compensation
|
Yes
|
|
N2 or other inert gas supply for cell pressurization
|
Required (gas supply and regulator supplied by customer, more information on request)
|
|
Typical sample concentration range
|
0.01-10 mg/mL (sample dependent)
|
|
Number of samples (capacity)
|
6 x 96 well plate. Each scan requires 2 wells – total of 288 samples.
|
|
Sample storage temperature range
|
4 °C – 40 °C
|
*More details available on product specification sheet, available from Malvern Panalytical
Conclusion
Integrated with many new features, the MicroCal PEAQ-DSC Automated systems make DSC even easier to use and generate reproducible and reliable data. These systems also include tools for using DSC in a regulated environment.
Innovative tools, such as PEAQ-Smart, PEAQ-Compliance, PEAQ-Finder, PEAQ-Compare, and PEAQ-Performance together with the new hardware, make the latest MicroCal PEAQ-DSC Automated system an essential component of a biophysical toolbox in biopharmaceutical laboratories, CMOs and CROs, and core facilities at government and academic research laboratories.
References
- Gabrielson, J.P., and Weiss, W.F.J. Pharm. Sci. 104, 1240-1245 (2015) doi:10.1002/jps24393
- http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm495891.pdf
About Malvern Panalytical

Malvern Panalytical provides the materials and biophysical characterization technology and expertise that enable scientists and engineers to understand and control the properties of dispersed systems.
These systems range from proteins and polymers in solution, particle and nanoparticle suspensions and emulsions, through to sprays and aerosols, industrial bulk powders and high concentration slurries.
Used at all stages of research, development and manufacturing, Malvern Panalytical’s materials characterization instruments provide critical information that helps accelerate research and product development, enhance and maintain product quality and optimize process efficiency.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.