This article shows how a combination of nanoparticle tracking analysis (NTA) technique and a gold labeling method can be used to rapidly measure the concentration and size of Adeno-Associated Virus (AAV).
When AAV capsids were labeled with small gold nanoparticles, the amount of light scattered by each capsid was successfully increased, allowing rapid NTA characterization to determine AAV titer and aggregation state. Before gold labeling, NTA characterization of AAV was not possible. This novel technique should be applied to other types of small viruses (below 35nm diameter), assuming the surface chemistry is similar.
The NTA characterization also enables simultaneous measurements of concentration and size, both as a check of aggregation state and as a supplement/alternative to conventional titer assays. Various formulations have differing stability that may have a major effect on the performance of final products, but this stability cannot be sufficiently measured with traditional and existing techniques.
Overview of NTA
Both Brownian motion and light scattering properties are used by the NTA technique to acquire the samples’ particle size distribution in liquid suspension. When a laser beam is allowed to pass through the sample chamber, suspended particles in the path of this laser beam scatter light in such a way that they can be easily viewed through a 20x magnification microscope on which a camera is mounted.
This camera, operating at about 30fps (frames per second), records a video file of these particles moving under Brownian motion within the 100μm x 80μm x 10μm field of view (Figure 1).
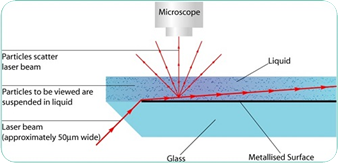
Figure 1. Schematic of NanoSight NTA optical configuration.
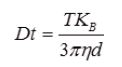
Equation 1. Stokes-Einstein equation.
It must be noted that NTA is not an ensemble technique that interrogates a large number of particles, but instead each particle is individually sized, regardless of the others. Figures 2 and 3 illustrate an example of the size distribution profile created by the NTA technique.
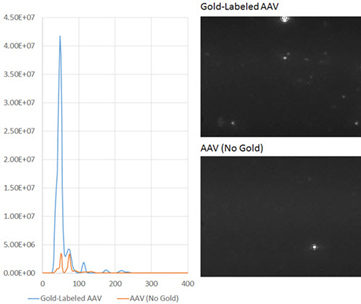
Figure 2. (right, top): Gold-labeled AAV NTA results; (right. lower): AAV NTA results without gold.
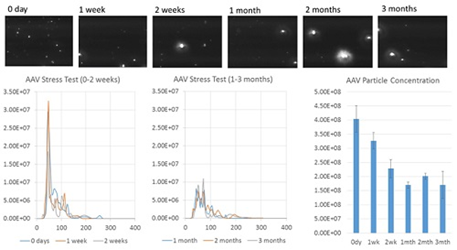
Figure 3. Au-AAV NTA results for AAV at different stages of a thermal stress test.
Moreover, the movement of the particles are measured within a fixed field of view (about 100μm x 80μm) that is illuminated by a beam of 10μm in depth. This helps estimate the sample’s scattering volume.
A concentration estimation, either as an overall total or in terms of particles per mL for any given size class, can be achieved by measuring the concentration of the particles within this field of view and extrapolating to a higher volume.
NTA’s lower sizing limit is related to the refractive index of the particle compared to the surrounding media. 10nm is the lower sizing limit for high scattering materials such as silver and gold nanoparticles, while 30-40nm is the lower sizing limit for biological particles and other similar low scattering materials.
Adeno-Associated Virus (AAV)
AAV is a small virus that measures roughly 25nm in diameter [1]. Gene therapy researchers often use this virus as a viral vector, thanks to its many attractive properties, such as low pathogenicity [2], ability to infect non-dividing cells [3], ability to integrate into a particular location on human chromosome 19 [4], and its tunable tropism towards different types of tissues [5]. To date, more than 130 clinical trials across the globe have used AAV as a viral vector [6].
Also, AAV is a well-defined virus with a well-known crystal structure [7]. Three types of capsid proteins (VP1, VP2, and VP3) are included in the AAV capsid in a 1:1:10 ratio. These proteins form five subunits to produce a total of 60 capsid proteins, giving rise to an icosahedral symmetry.
Though AAV has a known crystal structure, gene therapy researchers still find it difficult to rapidly measure the concentration of AAV particles. In the 1950s, the plaque assay was initially developed to measure virus titer [8] and it is now being used in academic laboratories. However, the plaque assay takes a considerable amount of time, requiring days or even weeks to complete.
To estimate AAV titer, modern methods such as quantitative polymerase chain reaction (qPCR) [9] and colorimetric assays like enzyme-linked immunosorbent assay (ELISA)[10], have been adopted by the industry to measure capsid proteins and genome counts.
However, while these techniques are better than the plaque assay, they still take a long time to complete. Therefore, a suitable method is required that can directly and rapidly measure the concentration of AAV particles.
Results of gold-labeled AAV NTA
The unlabeled AAV virus is very small, and therefore does not scatter sufficient amounts of light for NTA characterization. However, a new method in which gold nanoparticles are used to label the virus surface has enabled the AAV to be characterized by NTA.
This method takes advantage of the electrostatic attraction between the viral capsid and a highly scattering material, such as gold nanoparticles, which results in gold-labeled virus particles that scatter sufficient light and so can be viewed and tracked by the optical system. As a result, the size and concentration of AAV can now be measured using NTA.
A set of AAV samples were determined with this method. To compare the concentration determined by the NTA technique to the predicted titer, a highly-purified gold-labeled sample was considered.
A size distribution, with a particle concentration of 3.55 x 108 particles/mL and a primary peak, was produced by the gold-labeled AAV, representing only a 22% deviation from the predicted concentration of 4.5 x 108 particles/mL, based on a 100,000-fold dilution from the AAV titer of 4.5 x 1013 measured by qPCR (Figure 2, right, top).
A control experiment using identical conditions but without the use of gold labeling was carried out. In the resulting size distribution, it was observed that the measured concentration was much lower (Figure 2B) and the primary peak was not detected.
The small number of particles that were observed in the unlabeled AAV sample were most probably virus aggregates. Further control experiments were performed which showed that the primary peak recorded in the gold-labeled sample was not because of the presence of gold nanoparticle aggregates or background particles from the AAV vehicle buffer (data not shown).
AAV samples that were subjected to a thermal stress test for a period of three months (Figure 3), were then analyzed. Next, six AAV samples were heated to a temperature of 37°C for: 0 days, 1 week, 2 weeks, 1 month, 2 months, and 3 months, and stored at -20°C. On the day of analysis, the samples were thawed and labeled with gold before NTA characterization.
The results demonstrate a decrease in particle concentration and an increase in polydispersity over time. A primary peak indicating monodisperse AAV is seen for 2 weeks, but this peak starts to diminish within 1 month. The results show that for up to 1 week, AAV is, in general, stable at 37°C but after 2 weeks, displays significant signs of aggregation.
However, measurements of size distribution and concentration do not show a major change between 1 and 3 months, indicating that over that time period, AAV does not form larger aggregates. These results demonstrate the usefulness of the NTA gold-labeling characterization method to measure AAV concentration as well as formulation stability and aggregation state.
Conclusion
NTA characterization of AAV was enabled by using the electrostatic attraction between AAV and gold nanoparticles. For the first time, concentration of AAV particles is measured in minutes rather than hours or days.
This method also allows a direct measurement of the size and concentration of particles and requires no assumptions regarding AAV genome loading efficiency, capsid protein integrity, infection efficiency, or aggregation state.
References
- Schuster et al. Overcoming the Cystic Fibrosis Sputum Barrier to Leading Adeno-associated Virus Gene Therapy Vectors. Molecular Therapy (2014) 22(8), 1484–1493.
- Sun et al. Immune responses to adeno-associated virus and its recombinant vectors. Gene Therapy (2003) 10, 964–976.
- Podsakoff et al. Efficient gene transfer into nondividing cells by adeno-associated virus-based vectors. J Virol.(1994) 68(9), 5656–5666.
- Hüser et al. Adeno-associated virus integrates site-specifically into human chromosome 19 in either orientation and with equal kinetics and frequency. J Gen Virol. (2003) 84(1), 133-137.
- Zincarelli et al. Analysis of AAV Serotypes 1–9 Mediated Gene Expression and Tropism in Mice After Systemic Injection. Molecular Therapy (2008) 16(6), 1073–1080.
- Gene Therapy Clinical Trials Worldwide. The Journal of Gene Medicine. (2015) http://www.abedia.com/wiley/vectors.php
- Xie et al. The atomic structure of adeno-associated virus (AAV-2), a vector for human gene therapy. PNAS(2012) 99(16), 10405-10410.
- Dulbecco et al. Some problems of animal virology as studied by the plaque technique. Quant. Biol. (1953) 18, 273-279
- Rohr et al. Fast and reliable titration of recombinant adeno-associated virus type-2 using quantitative real-time PCR. J Virol Methods. (2002) 106(1), 81-88.
- Grimm et al. Titration of AAV-2 particles via a novel capsid ELISA: packaging of genomes can limit production of recombinant AAV-2. Gene Therapy. (1999), 6(7), 1322-1330.
About Malvern Panalytical

Malvern Panalytical provides the materials and biophysical characterization technology and expertise that enable scientists and engineers to understand and control the properties of dispersed systems.
These systems range from proteins and polymers in solution, particle and nanoparticle suspensions and emulsions, through to sprays and aerosols, industrial bulk powders and high concentration slurries.
Used at all stages of research, development and manufacturing, Malvern Panalytical’s materials characterization instruments provide critical information that helps accelerate research and product development, enhance and maintain product quality and optimize process efficiency.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.