Sponsored Content by SartoriusReviewed by Alex SmithMay 20 2022
Using a multiplex and high throughput serological assay to achieve a thorough profile of antibodies against SARS-CoV-2 enables researchers to advance their research on COVID-19.
Due to constant mutation and evasion, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) persists. There is, therefore, an ongoing need to accurately assess the duration and the number of antibody responses against SARS-CoV-2, particularly antibodies targeting the SARS-CoV-2 Spike protein receptor binding domain (RBD), as the development of humoral immune response against this virus is critical for protection.
By binding to the angiotensin-converting enzyme 2 (ACE2), the SARS-CoV-2 Spike RBD mediates viral entry into human cells and, as such, is the primary target of many COVID-19 vaccines in use today.
Antibodies against the SARS-CoV-2 Spike RBD tend to have potent viral neutralizing activity and correspond with protective immunity against the virus.
The three major isotopes (IgG, IgM and IgA) of antibodies specific for SAR-CoV-2 Spike RBD can be quantitated by serological testing and therefore are used to facilitate characterization and monitor the duration of antibody responses in both vaccinated individuals and COVID-19 patients, as well as to detect asymptomatic infections and review past infection prevalence in a population.
When it comes to detecting anti-SARS-CoV-2 Spike RBD IgG, IgM or IgA antibodies, there are several methods available, including a lateral flow immunoassay, flow cytometry, and traditional enzyme-linked immunosorbent assay (ELISA). However, each of these methods has limitations, such as:
- Provides solely qualitative results
- Low specificity and sensitivity
- Can be labor-intensive and may not necessarily help high-throughput applications
- Multiplexing analysis of IgG, IgM and IgA antibodies is not allowed
- Require large amounts of reagents
A robust, sensitive and rapid serological assay for the measurement of IgG, IgM and IgA anti-SARS-CoV-2 Spike RBD antibodies (in a single well of a 96 or 384-well plate with antibody detection levels in the pg/mL range) is provided by the iQue® SARS-CoV-2 (IgG, IgM and IgA) kit.
Time and precious patient samples are provided by a combined detection of all three antibody isotypes, which offers a dynamic, comprehensive profile over time of humoral immune responses after natural infection with SARS-CoV-2 or immunization with COVID-19 vaccines. Research can be accelerated using the iQue® SARS-CoV-2 (IgG, IgM and IgA) kit.
The assay concept
The iQue® SARS-CoV-2 (IgG, IgM and IgA) Kit is the name given to a bead-based immunoassay that has been designed for the simultaneous detection of human IgA, IgM, and IgG, IgM antibodies which are specific for SARS-CoV-2 Spike protein RBD produced by the immune system as the body’s response to vaccination or viral infection.
To achieve robust, multiplexed analysis on the iQue® platform with a VBR configuration, the iQue® SARS-CoV-2 (IgG, IgM and IgA) Kit has been carefully optimized. A streamlined workflow and pre-set templates have been designed for ease of use and to help accomplish a quantitative and qualitative analysis of anti-SARS-CoV-2 Spike RBD antibodies from blood samples (both sera and plasma).
Basic workflow:
- Adds pre-diluted patient samples to 96 or 384-well plate
- Adds SARS-CoV-2 Spike RBD-coupled Beads to capture anti-SARS-CoV-2 Spike RBD antibodies
- Adds SARS-CoV-2 Antibody Detection Cocktail containing fluorescently labeled anti-human IgG, IgM and IgA antibodies
- Analyzes the plate on the iQue® platform (VBR configuration).
Key advantages
- It allows for the accurate assessment of immune response by producing a simultaneous and sensitive measurement of anti-SARS-CoV-2 Spike RBD IgG, IgM and IgA antibodies which are induced in response to vaccination or natural infection.
- It allows the user to maximize their productivity – high-throughput analysis of the three major isotypes (IgG, IgM and IgA) of antibodies specific for SARS-CoV-2 Spike RBD is analyzed in a single well for the comprehensive analysis of the humoral immune response.
- Users can save precious samples – This streamlined workflow conserves precious samples - only requiring a single 10 µL sample diluted at least 100-fold.
Obtain an accurate assessment of immune response
The technology facilitates a simultaneous and sensitive measurement of anti-SARS-CoV-2 Spike RBD IgG, IgM and IgA antibodies which are induced in response to natural infection or vaccination.
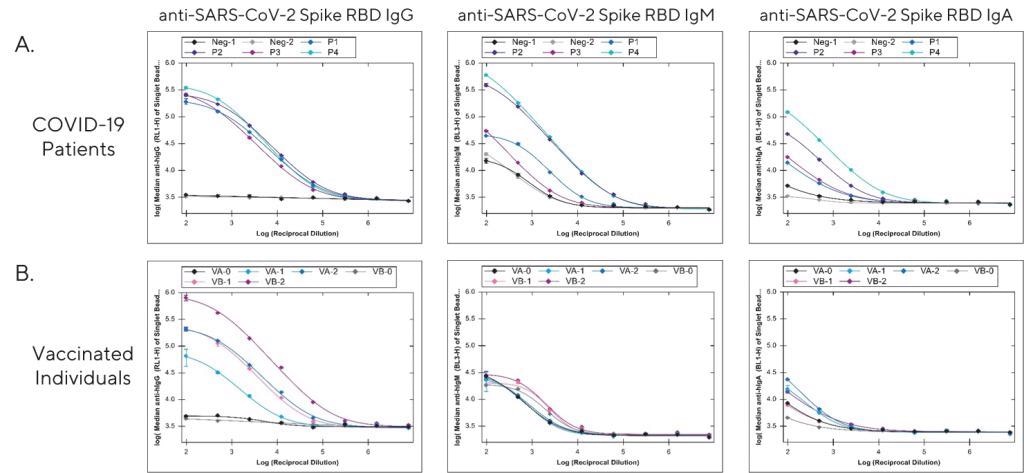
Figure 1. Analysis of anti-SARS-CoV-2 Spike RBD IgG, IgM, and IgA antibodies in (A) serum from recovered COVID-19 patients (P1-4). Serum collected from two individuals prior to the pandemic were included as negative controls (Neg. 1-2). (B) Analysis of serum collected from two individuals (VA & VB) prior to immunization (VA-0, VB-0), and again after the first (VA-1, VB-1) and second injection (VA-2, VB-2) with the BNT162b2 (Pfizer-BioNTech) COVID-19 vaccine. Serum samples were diluted 1:100 in Assay Diluent and then serially diluted 1:5 in triplicate to generate dilution curves. Image Credit: Sartorius
Maximize productivity
Multiplexed assay allows for the high throughput analysis of the three significant isotypes (IgG, IgM and IgA) of antibodies specific to SARS-CoV-2 Spike RBD in a single well for the thorough examination of the humoral immune response with novel visualization tools and real-time data analysis.
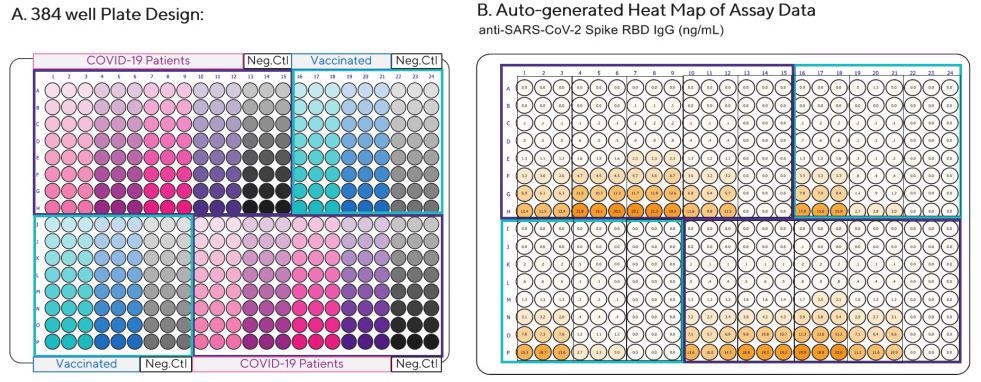
Figure 2. Analysis of anti-SARS-CoV-2 Spike RBD IgG, IgM, and IgA antibodies in serum from recovered COVID-19 patients or individuals vaccinated with the BNT162b2 (Pfizer-BioNTech) vaccine. Serum samples were diluted 1:100 in Assay Diluent and then serially diluted 1:3 in triplicate. Serum collected from two COVID-19 negative individuals were included as negative controls (Neg. Ctl). Each color represents triplicate dilution curves which were repeated in different quadrants of plate. (A) Plate layout (B) Heat Map of anti-SARS-CoV-2 Spike RBD IgG concentrations per well (ng/mL). Image Credit: Sartorius
Protect precious samples
Streamlined workflow conserves precious samples.
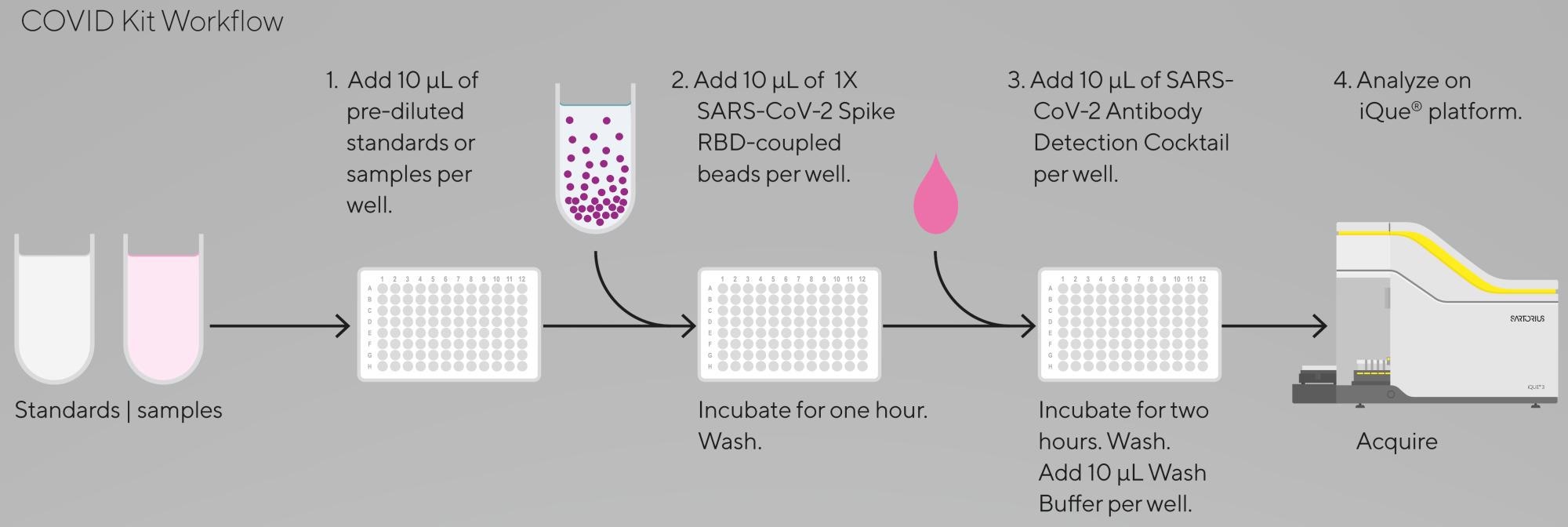
Figure 3. iQue® SARS-CoV-2 (IgG, IgM and IgA) Kit workflow. Easy to follow steps and low sample volumes needed (requires only a single 10 µL of plasma / serum diluted at least 100-fold). Image Credit: Sartorius
About Sartorius

Sartorius is a leading international pharmaceutical and laboratory equipment supplier. With our innovative products and services, we are helping our customers across the entire globe to implement their complex and quality-critical biomanufacturing and laboratory processes reliably and economically.
The Group companies are united under the roof of Sartorius AG, which is listed on the Frankfurt Stock Exchange and holds the majority stake in Sartorius Stedim Biotech S.A. Quoted on the Paris Stock Exchange, this subgroup is comprised mainly of the Bioprocess Solutions Division.
Innovative technologies enable medical progress
A growing number of medications are biopharmaceuticals. These are produced using living cells in complex, lengthy and expensive procedures. The Bioprocess Solutions Division provides the essential products and technologies to accomplish this.
In fact, Sartorius has been pioneering and setting the standards for single-use products that are currently used throughout all biopharmaceutical manufacturing processes.
Making lab life easier
Lab work is complex and demanding: Despite repetitive analytical routines, lab staff must perform each step in a highly concentrated and careful way for accurate results.
The Lab Products and Services Division helps lab personnel excel because its products, such as laboratory balances, pipettes and lab consumables, minimize human error, simplify workflows and reduce physical workloads
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.