Microglia — the resident immune cells of the central nervous system — have a crucial role to play in the control of tissue response to pathological or inflammatory insults or in the regulation of homeostasis.
Microglial cells influence the synaptic remodeling and turnover of dendritic spines via the removal of unnecessary or damaged synapses or neurons. A limited number of tools and in vitro model systems are available that help to optimize, track, and investigate morphological and functional changes of the microglial cells.
This article defines Rat Primary, iPSC-derived, and immortalized microglia and presents data assessing the potential of these cells to phagocytose apoptotic Neuro-2A (N2A) cells and pHrodo®-labeled Escherichia coli bioparticles. This is achieved by using a quantitative, live-cell imaging method with the Incucyte® S3 Live-Cell Analysis System for Neuroscience.
Quick guide
Phagocytosis/efferocytosis
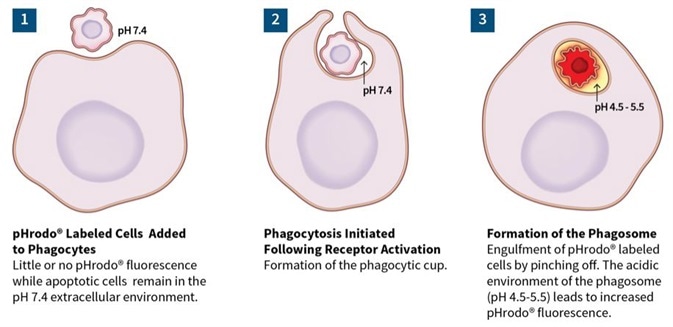
Image Credit: Incucyte®.
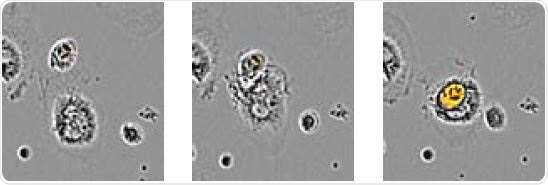
Image Credit: Incucyte®.
The above image shows time-lapse visualization of Axol BioScience’s iPSC-derived microglia engulfing pHrodo® Orange-labeled apoptotic N2A cells. Images confirmed the penetration of an apoptotic target cell into the microglia’s cytoplasm.
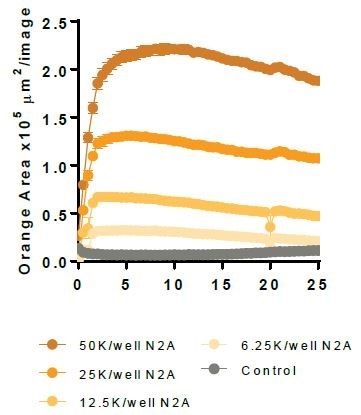
Primary microglial efferocytosis of apoptotic Neuro-2a cells
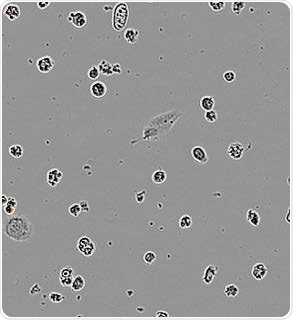
Apoptotic N2A alone. Image Credit: Incucyte®.
Microglia with N2A. Image Credit: Incucyte®.
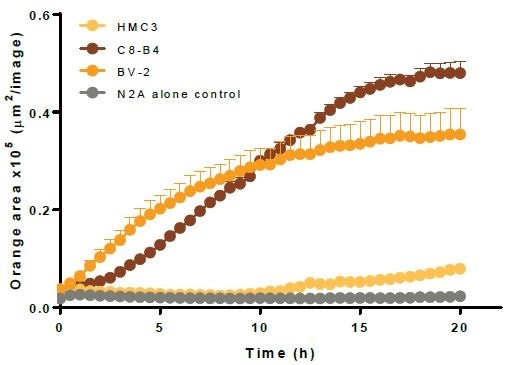
Image Credit: Incucyte®.
N2A cells were first pre-treated with staurosporine for a period of 24 hours, then labeled with the IncuCyte® pHrodo® Orange Cell Labeling Kit, and finally added to pre-plated primary rat microglia (Brain Bits, 20,000 K per well). N2A cells alone were observed to have minimal fluorescence (refer to the above image).
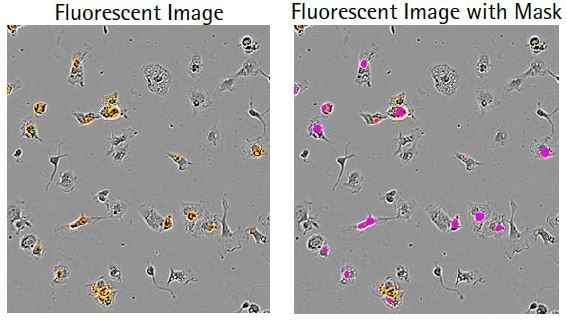
Microglial cells engulf the labeled apoptotic N2A cells, which results in increased orange fluorescence (florescent image) and this is sectioned using the automated Incucyte® Live-Cell Analysis Software (fluorescent image with mask). Following this, a kinetic N2A density-dependent response is measured over time (refer to the bottom image).
Characterization of immortalized microglia efferocytosis
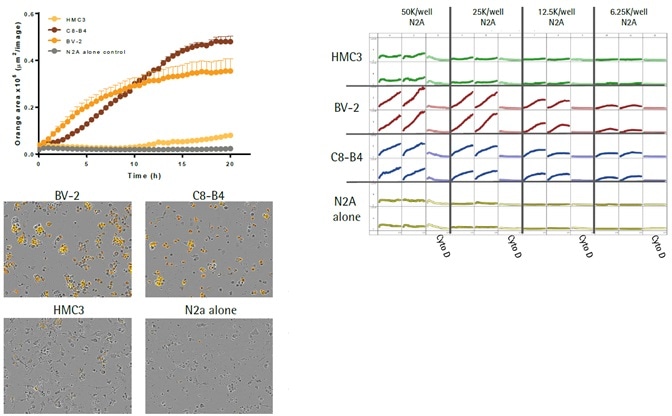
Image Credit: Incucyte®.
- The top left image shows kinetic efferocytosis response of the engulfment of N2A cells for three different immortalized microglial cell lines—BV2, C8-B4, and HMC3. C8-B4 and BV-2 cells displayed an increasing signal over time, whereas HMC3 cells showed minimal efferocytosis.
- The top right image shows microplate graph of the kinetic efferocytosis response from a full 96-well plate. C8-B4, BV2, and HMC3 cells were seeded on the same plate at 5K cells per well. This was followed by adding pHrodo®-labeled apoptotic N2A cells at reducing densities from left to right. To suppress efferocytosis, cytochalasin D was added at every N2A density.
- Images captured at 20 hours revealed the fluorescent signal from C8-B4 and BV-2 cells and also revealed the absence of signal from HMC3 cells over the control apoptotic N2A cells alone (refer to the bottom-left image).
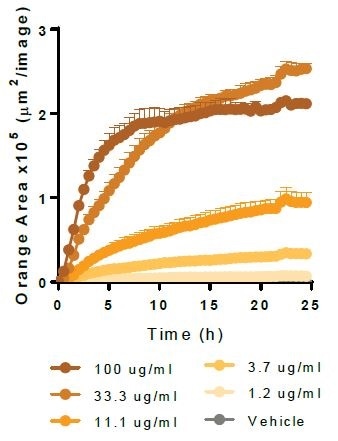
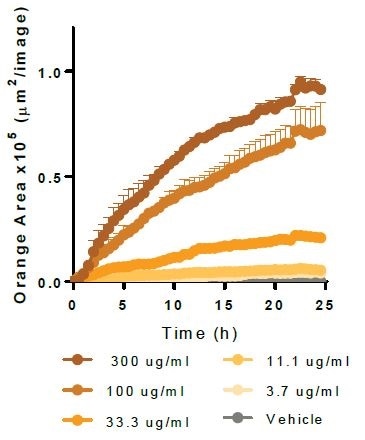
Effect of cytochalasin D and cilengitide on microglial function
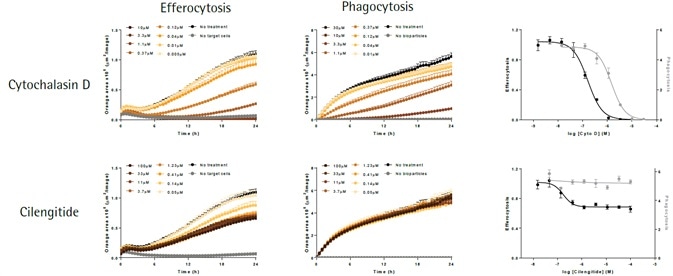
Image Credit: Incucyte®.
The apoptotic N2A cells (shown in left-hand panels) or E. coli bioparticles (shown in middle panels) are efferocytosed by the BV-2 effector cells (20,000K per well). Cytochalsin D (shown in top panels) triggers a concentration-dependent suppression of both phagocytosis and efferocytosis, yielding IC50 values of 1.5 μM and 0.16 μM, respectively.
Cilengitide—an inhibitor of αVβ5 and αVβ3 integrins—selectively attenuates efferocytosis (IC50 value 0.17 μM), with least impact on phagocytosis at maximum concentration tested (100 μM). While such data supports the role of integrins in the cellular interactions needed for efferocytosis, it does not do so in the phagocytosis of bacteria-based bioparticles.
Incucyte® S3 Live-Cell Analysis System for Neuroscience
Image Credit: Incucyte®.
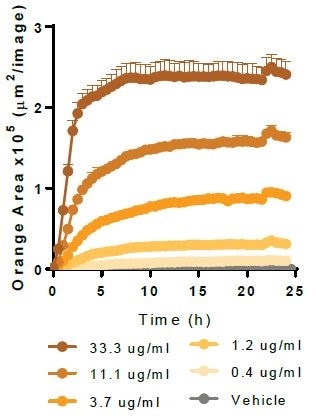
iPSC-derived microglial phagocytosis of relevant target material
Apoptotic Neuro-2A. Image Credit: Incucyte®.
Beta-Amyloid (1-42). Image Credit: Incucyte®.
Alpha-Synuclein. Image Credit: Incucyte®.
Myelin Basic Protein. Image Credit: Incucyte®.
Physiologically relevant target material is phagocytosed by Axol Biosciences’ iPSC-derived microglia (30,000 K per well). Kinetic graphs show the concentration-dependent reaction to pHrodo®-labeled apoptotic Neuro-2A cells, Myelin Basic Protein, Alpha-Synuclein, and Beta-Amyloid (l-42).
Effect of LPS activation on microglial cell line function
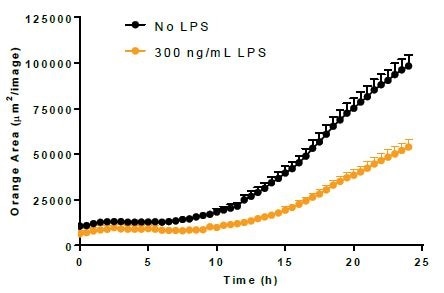
Efferocytosis. Image Credit: Incucyte®.
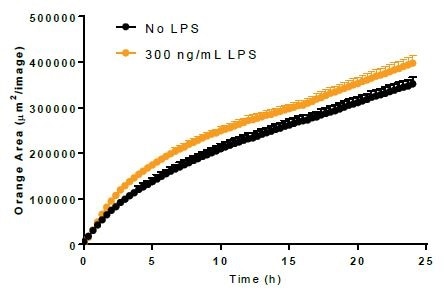
Phagocytosis. Image Credit: Incucyte®.
- Using 300 ng/mL LPS, BV-2 cells were incubated for a period of 24 hours before adding either bioparticles or Incucyte® pHrodo®-labeled N2A cells to induce the activation of microglia.
- LPS treatment minimally increased the phagocytosis of bioparticles and decreased the efferocytosis reaction to apoptotic N2A cells.
Differential morphology of microglial sources
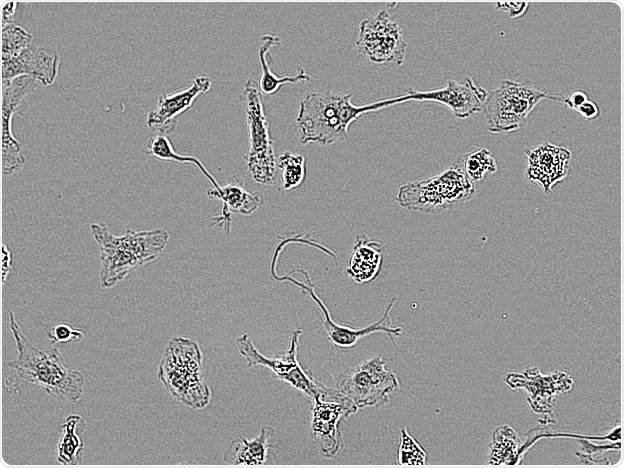
Rat Primary microglia. Image Credit: Incucyte®.
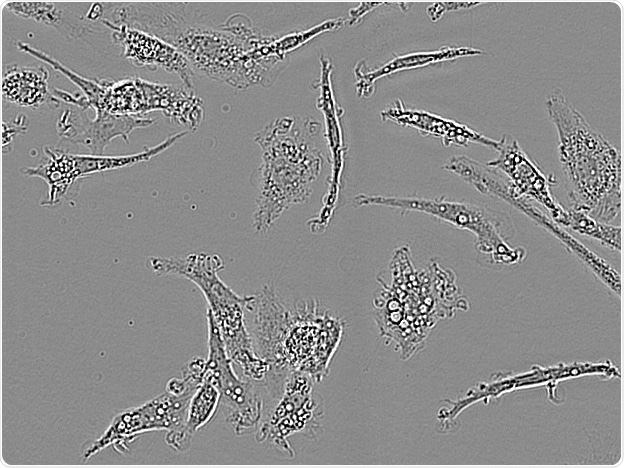
Axol IPSC-derived microglia. Image Credit: Incucyte®.
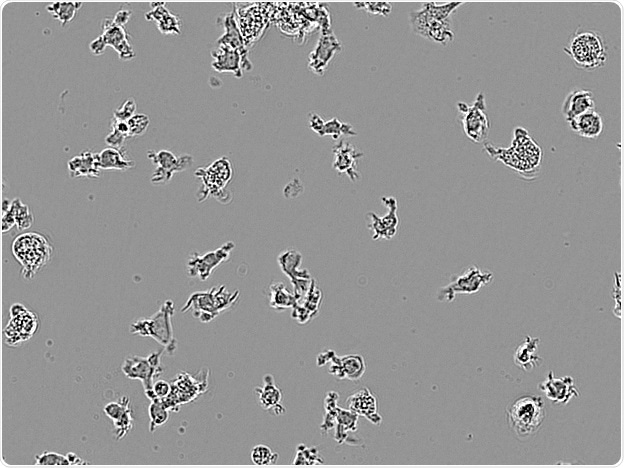
CDI IPSC-derived microglia. Image Credit: Incucyte®.
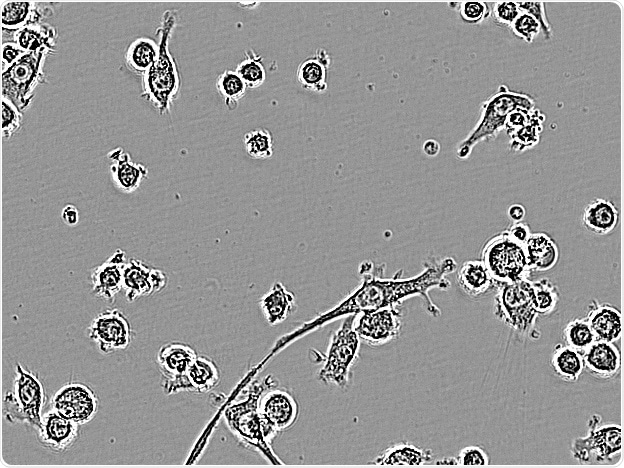
BV-2. Image Credit: Incucyte®.
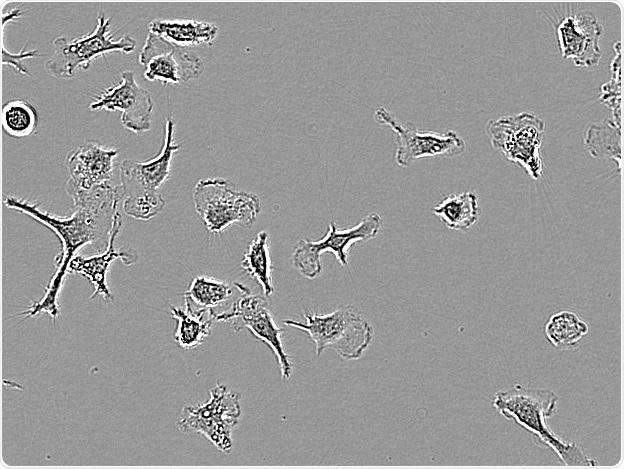
C8-B4. Image Credit: Incucyte®.
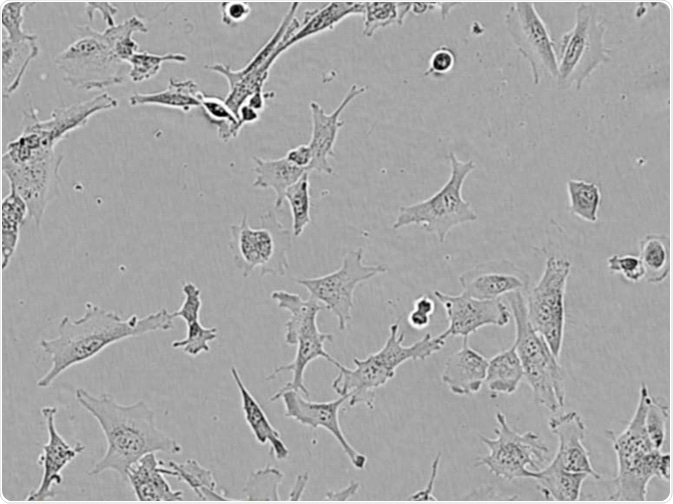
HMC3. Image Credit: Incucyte®.
Microglial cells from a wide range of sources were defined for morphological phenotype (20x zoomed to same scale).
Shown above are BrainBits’ representative images from Rat Primary microglia (top left), Axol BioScience’s iPSC-derived microglia (top middle), Cellular Dynamics International’s iPSC-derived microglia (top right), immortalized murine microglia cell lines BV-2 (bottom left), C8-B4 (bottom middle), and HMC3 human immortalized microglia (bottom right). Based on the source and species, differential morphology was seen.
Conclusions
- Microglia can have highly different morphology in vitro based on the species as well as the source of the cells.
- Microglia can phagocytose a wide range of targets such as apoptotic N2A cells, E. coli bioparticles, and physiologically relevant protein targets.
- Immortalized microglia have variable degrees of phagocytic potential, and this can be altered by LPS-mediated activation.
- Efferocytosis and phagocytosis are distinct processes that can be selectively suppressed by pharmacological means.
- The Incucyte® S3 Live-Cell Analysis system for Neuroscience can be utilized with the pHrodo® Orange Cell Labeling Kit to define morphology and measure functional efferocytic and phagocytic activity in microglia.
Acknowledgments
Produced from materials originally authored by Aaron C. Overland, John N. Rauch, Libuse Oupicka, Michael L. Bowe, Susana L. Alcantara, Gillian Lovell, Time Dale and Daniel M. Appledorn from Essen BioScience.
About Incucyte®
The Incucyte® Real-Time Quantitative Live Cell Analysis System, designed by Essen BioScience, Inc (now a Sartorius company) is the first system to continuously quantify cell behavior over time (from hours to weeks) while cells remain undisturbed inside a standard incubator. The Incucyte® System automatically collects and analyzes images continuously around the clock, providing insight into active biological processes that is difficult to achieve with endpoint assays. The Incucyte® suite of assays utilize proprietary reagents that do not perturb cell health with validated 96/384 well format protocols that save time and money.
Incucyte® Live-Cell Analysis has revolutionized numerous studies, with applications such as stem cell monitoring and reprogramming, label-free analysis, ATP metabolism, multiplexing, neurite outgrowth and dynamics, migration/invasion, 3D-spheroids, angiogenesis, NETosis, reporter gene expression, immunocytochemistry, immune cell killing, apoptosis, cytotoxicity, kinetic data generation, antibody internalization. All this without the removal of cells from an incubator.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.