Sponsored Content by SartoriusReviewed by Alex SmithApr 28 2022
NK cell-related immunotherapeutics, such as tumor-specific monoclonal antibodies (mAbs) to elicit NK cell-mediated ADCC against tumor cells, are being developed for the treatment of cancers. As an example, Rituximab and obinutuzumab, two mAbs that target CD20, have long been used to treat varied B cell malignancies.
Rituximab is a type I, chimeric anti-CD20 mAb (mouse variable region + human IgG1 Fc region), whereas obinutuzumab is a type II, fully humanized IgG1 mAb that adheres to a partly overlapping, but distinct epitope on CD20 compared to rituximab and was glycoengineered to decrease fucosylation in the Fc region, resulting in improved binding affinity for FcγRIIIa (CD16a) which enhanced ADCC.1–3
To determine the prospective clinical effectiveness of newly designed tumor-specific mAbs, NK cell-mediated ADCC must be assessed. Donor cell variability, on the other hand, can influence the degree of NK cell-mediated ADCC. As a result, in addition to determining cytolytic activity, the number and activation state of the donor NK cells must be determined.
Conventional cytotoxicity assays are time-consuming, and characterizing donor effector cells necessitates additional downstream assays.
Anti-hCD20 mAbs, similar to rituximab and obinutuzumab, were used in this study to see if a multiplexed, high throughput assay that measures target cell killing, expression of NK cell phenotypic markers, and effector protein secretion could be used to distinguish between mAbs’ ADCC potency and provide insight into their potential treatment efficiency across donors.
Experimental approach
Workflow
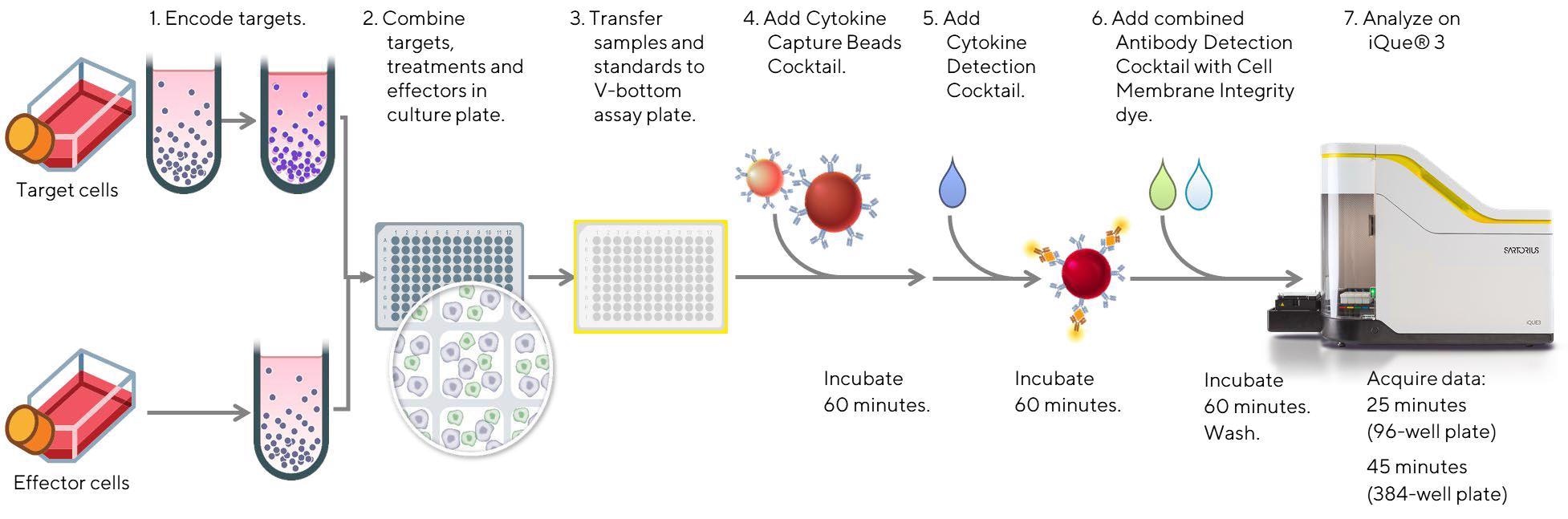
Figure 1. Schematic diagram of the workflow for the analysis of NK cell cytotoxic function, along with assessment of the NK cell activation state and cytokine/effector protein release using the iQue® Human NK Cell Killing Kit. Effector cells (Cellero; human PBMCs or enriched, negatively selected human NK cells) were thawed and allowed to recover in media (Corning; RPMI 1640 medium with 10% fetal bovine serum for enriched NK cells, or RPMI 1640 containing 10% FBS, 1% non-essential amino acids, 1% sodium pyruvate, and 1% Pen/Strep for PBMCs) for 16-18 h overnight. On the assay day, a frozen aliquot of CD20+ Raji tumor target cells (ATCC, Burkitt’s lymphoma cell line) previously stained with a fluorescent encoder dye, was thawed, counted, and plated in triplicate in a 96 well plate at 20 K/well for 30 minutes at room temperature in the presence of individual anti-CD20 mAbs at concentrations ranging between 0- 10 µg/mL. The anti-hCD20 mAbs (InvivoGen) included hcd20-mab1 (Rtx-G1) composed of a human IgG1 Fc region and variable region of rituximab, hcd20ga-mab1 (Ob-G1) which was a fully humanized mAb with the variable region of obinutuzumab and a native human IgG1 Fc region, and hcd20ga-mab13 (Ob-G1nF) with the variable region of obinutuzumab and a nonfucosylated human IgG1 Fc region. Another anti-hCD20 mAb, hcd20ga-mab7 (Ob-A2), was used as a negative control. It also possessed the variable region of obinutuzumab, but had a human IgA2 constant region, and did not bind FcγRIIIa (CD16a). Next, effector cells were added at an Effector to Target (E:T) ratio of 10:1 (PBMCs) or 1:1 (enriched NK cells). In parallel, effector cells were cultured in triplicate in media alone, or cocultured with targets alone to evaluate direct tumor cell killing without antibody. Direct antibody-mediated killing of tumor cells was also assessed by culturing Raji cells with anti-CD20 mAbs in the absence of effector cells. Following co-culture for 4 hours at 37 °C, 5% CO2, 10 µL samples were assayed using the multiplexed iQue® Human NK Cell Killing Kit plus iQue® Human NK Companion Kits, and data acquisition was performed on the iQue® platform (VBR configuration) for advanced flow cytometry. Image Credit: Sartorius
Simultaneous endpoint measurement in a single well
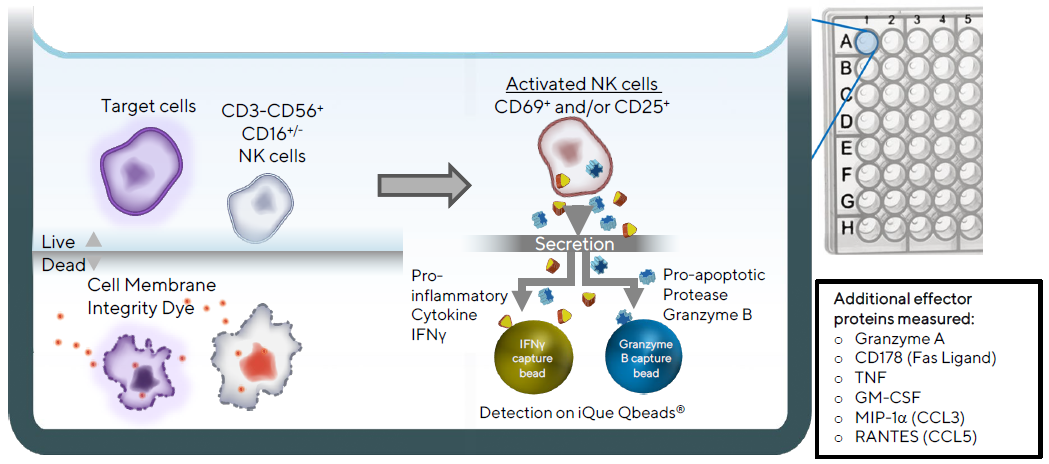
Figure 2. Illustration of iQue® Human NK Cell Killing Kit assay principles. Target cells are distinguished from effector cells by staining with a fluorescent encoder dye, and tumor cell killing is then determined by staining with a fluorescent cell membrane integrity dye. NK cells are identified using CD3, CD56, and CD16. Their activation state is assessed using CD69 and CD25. Production of the pro-inflammatory cytokine, Interferon gamma (IFNγ), and the pro-apoptotic serine protease, Granzyme B, are quantified using a 2-plex iQue Qbeads® in a sandwich immunoassay format in the same well. Additional compatible cytokines and effector proteins are measured by incorporating other iQue® Human NK Cell Companion Kits into the assay. Image Credit: Sartorius
Results
The level of ADCC is mAb and donor-dependent
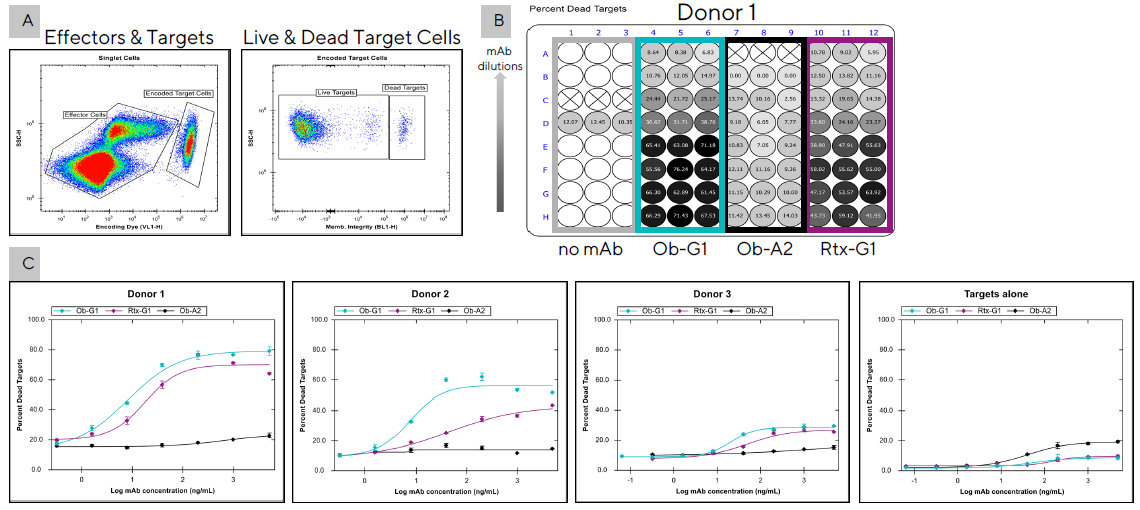
Figure 3. Comparison of the ADCC potency of two anti-hCD20 mAbs among three different donors. PBMCs (200K/well) from three separate donors were co-cultured in triplicate with encoded Raji tumor cells (20K/well) in the presence of different anti-hCD20 mAbs (Ob-G1, Rtx-G1, or Ob-A2, as a negative control). After 4h, 10 µL samples were transferred to assay plates and analyzed using the iQue® Human NK Cell Killing Kit plus iQue® Human NK Cell Companion Kits. (A) Dot plot showing separation of target cells from effector cells with the use of a fluorescent encoder dye. Target cell killing was then determined with the use of a cell membrane integrity dye to distinguish live and dead targets cells. (B) Percent dead targets displayed as a plate heat-map for Donor 1 over serial dilutions of 3 mAbs starting at 10 µg/mL. (C) EC50 curves showing percent tumor cell killing by effector cells from three donors tested in a second experiment with 3 mAbs over five-fold serial dilutions starting at 5 µg/mL. Raji cells were also incubated with the mAbs alone to evaluate direct Ab-mediated cytotoxicity (Targets alone). Image Credit: Sartorius
Table 1. Comparison of mAb EC50 in relation to donor NK cell phenotype and FcγRIIIa genotype. Source: Sartorius
| Donor ID |
FcRγIIIa 158 genotype |
% NK cells (CD3-CD56+) |
% CD16+ NK Cells |
Direct NK cell Killing (%) |
mAb EC50 (ng/mL) |
| Rtx-G1 |
Ob-G1 |
| Donor 1 |
V/V |
14.6 |
81 |
7 |
18 |
8 |
| Donor 2 |
V/F |
8 |
82 |
3 |
43 |
8 |
| Donor 3 |
F/F |
14.6 |
44 |
5 |
50 |
20 |
Effector protein secretion levels correspond with mAb potency
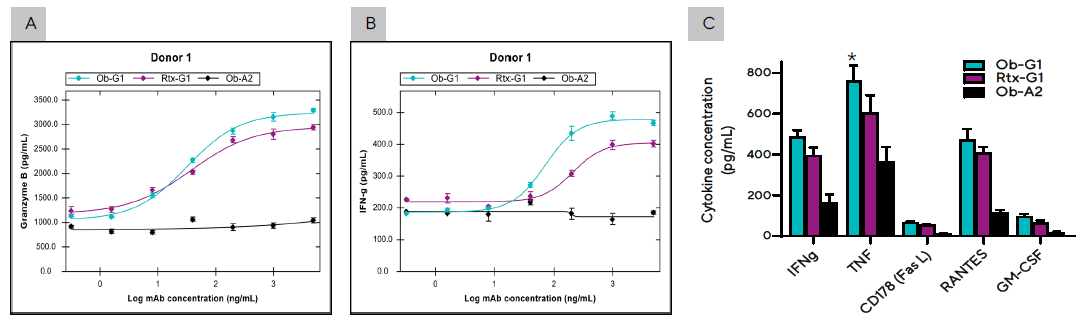
Figure 4. Effector proteins and cytokines secreted during the PBMC- Raji tumor cell co-culture described in Figure 3C. Secretion levels of (A) Granzyme B and (B) IFNγ by PBMCs from Donor 1 cultured with Raji tumor cell in the presence of anti-hCD20 mAbs at concentrations ranging between 0–5 µg/mL. (C) Summary of cytokines produced by Donor 1 PBMCs co-cultured with Raji cells and 3 different mAbs at 1 µg/mL dose. Bar graph data presented as Mean +/- 1 SD, 3 replicates/mAb dose. (*) Significantly higher levels of cytokines produced in co-culture using ObG1 compared to Rtx-G1 anti-CD20 mAb, p < 0.05. Image Credit: Sartorius
Donor ADCC differences are reflected in effector protein levels
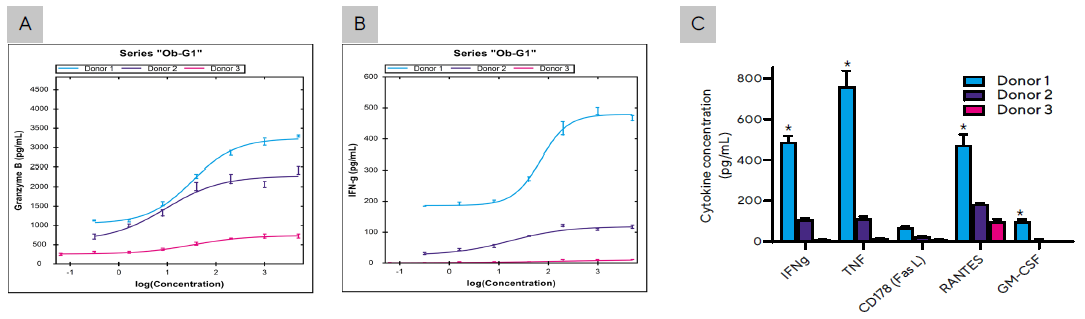
Figure 5. Secretion levels of (A) Granzyme B and (B) IFNγ by PBMCs from all 3 donors cultured with Raji tumor cells as described in Figure 3C at Ob-G1 concentrations ranging between 0–5 µg/mL. (C) Summary of cytokines produced by PBMCs from three donors co-cultured with Raji cells in the presence of Ob-G1 mAb at 1 µg/mL. Bar graph data presented as Mean +/- 1 SD, 3 replicates/mAb dose. (*) Significantly higher levels of cytokines produced by Donor 1 compared to Donor 2 or 3; p < 0.05. Image Credit: Sartorius
ADCC and cytokine production were enhanced using a non-fucosylated anti-CD20 mAb with either PBMCs or enriched NK cells as effector cells
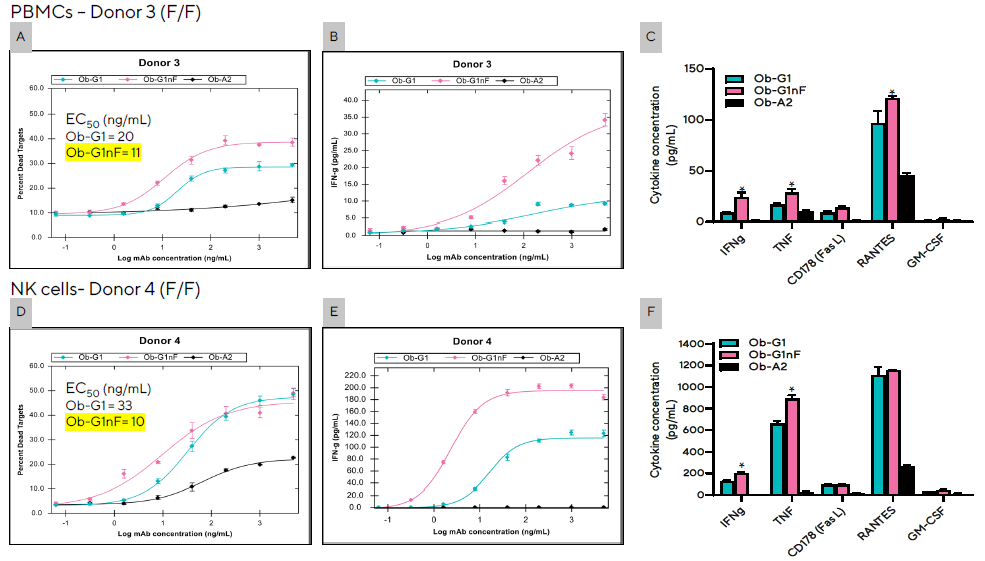
Figure 6. The degree of NK cell-mediated ADCC and secretion levels of effector proteins were enhanced with the use of a non-fucosylated antiCD20 mAb (Ob-G1nF). Encoded Raji tumor cells (20K/well) were co-cultured with PBMCs (A, B, C) from Donor 3 at an E:T of 10:1, or enriched NK cells (D,E,F) from Donor 4 at an E:T of 1:1 in the presence of Ob-G1, Ob-G1nF, or Ob-A2 anti-hCD20 mAbs at concentrations ranging between 0–5 µg/mL. Both donors had a FcγRIIIa-158 F/F genotype. Percent tumor cell killing (A and D), and production of IFNγ (B and E) following coculture for 4 hours. (C and F) Summary of cytokines produced in the presence of 3 different mAbs at 1 µg/mL dose. Bar graph data presented as Mean +/− 1 SD, 3 replicates/mAb dose. (*) Significantly higher levels of cytokines produced in co-culture when using anti-CD20 mAb Ob-G1nF compared to Ob-G1, p < 0.05. Image Credit: Sartorius
Conclusion
The iQue® Human NK Cell Killing Kit enabled the simultaneous determination of NK-mediated ADCC, expression of NK cell phenotypic markers, and estimation of IFNγ and Granzyme B, as well as the flexibility to quantify up to six additional effector proteins and cytokines using the iQue® Human NK Cell Companion Kits in the same well.
The Ob-G1 anti-hCD20 mAb displayed improved ADCC potency compared to Rtx-G1 for all donors tested, according to the findings of this study.
The use of the non-fucosylated anti-mAb, Ob-G1nF, with either PBMCs or enriched NK cells resulted in an even greater increase in ADCC and cytokine secretion. When the negative control anti-hCD20 mAb, Ob-A2, was added to the co-cultures, there was no increase in ADCC above baseline, and only very low levels of direct killing of Raji tumor cells were observed when the anti-hCD20 mAbs were used alone.
Donor 1’s PBMCs with a FcγRIIIa-158 V/V genotype had higher ADCC than Donor 2’s (FcγRIIIa-158 V/F) or Donor 3’s (FcγRIIIa-158 F/F) either using the Ob-G1 or Rtx-G1 anti-hCD20 mAbs. Furthermore, the levels of secretion of many effector proteins and cytokines mirrored the level of ADCC evoked by the various mAbs tested, and the variation in ADCC observed between donors.
On the whole, this research revealed that using the iQue® Human NK Cell Killing Kit in combination with the iQue® Human NK Cell Companion Kits allows for a quick evaluation of the potency of various mAbs in ADCC mediated by NK cells, as well as a clear distinction between donors’ mAb efficacy.
Furthermore, analyzing NK-cell surface marker expression and cytokine secretion at the same time may reveal potential mechanistic distinctions that contribute to the differences in tumor cell killing noted between donors.
With a short assay time of only 3.5 hours, the assay requires only 10 µl of sample and measures multiple endpoints from the same effector + target co-culture. The results could also be visualized right away, thanks to the iQue® platform and iQue Forecyt® software.
As a result, the multiplexed iQue® Human NK Cell Killing Kit combines traditional workflows into a single assay platform, facilitating the creation and evaluation of new NK cell-related cancer immunotherapies.
Key advantages
- Conventional workflows are consolidated into a single rapid, miniaturized, easy-to-use multiplex assay that requires minimal sample manipulation and can be run in 96 or 384-well plates
- Simultaneously measures the expression of NK cell surface proteins and cytokines in connection to target cell killing
- Low sample volumes are required, allowing valuable samples to be saved
- Real-time data analysis and novel visualization tools are possible with the iQue® platform and the embedded iQue Forecyt® Software
References
- Salles G., et al., Adv Ther. 2017;34(10), 2232–2273
- Tobinai K, et al., Adv Ther. 2017;34(2):324-356.
- Mössner E, et al., Blood. 2010;115(22):4393-402.
About Sartorius

Sartorius is a leading international pharmaceutical and laboratory equipment supplier. With our innovative products and services, we are helping our customers across the entire globe to implement their complex and quality-critical biomanufacturing and laboratory processes reliably and economically.
The Group companies are united under the roof of Sartorius AG, which is listed on the Frankfurt Stock Exchange and holds the majority stake in Sartorius Stedim Biotech S.A. Quoted on the Paris Stock Exchange, this subgroup is comprised mainly of the Bioprocess Solutions Division.
Innovative technologies enable medical progress
A growing number of medications are biopharmaceuticals. These are produced using living cells in complex, lengthy and expensive procedures. The Bioprocess Solutions Division provides the essential products and technologies to accomplish this.
In fact, Sartorius has been pioneering and setting the standards for single-use products that are currently used throughout all biopharmaceutical manufacturing processes.
Making lab life easier
Lab work is complex and demanding: Despite repetitive analytical routines, lab staff must perform each step in a highly concentrated and careful way for accurate results.
The Lab Products and Services Division helps lab personnel excel because its products, such as laboratory balances, pipettes and lab consumables, minimize human error, simplify workflows and reduce physical workloads
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.