Introduction
The growth and function of cells in culture can be altered by several variables. Since many sources of variability are inherent to the stochastic processes in biological systems, they are largely uncontrollable [1]. However, some factors can be identified and controlled.
The controllable factors include poor CO2 incubator performance, because of lack of calibration and stability of humidity, CO2, and temperature over time; and inconsistent and/or non-quantitative techniques for feeding and splitting cell cultures before running cell-based assays, such as inconsistent limits of feeding schedules and cell density, changes in cell morphology, and inconsistent cell density at the time of assay.
The third key controllable factor is alterations in media constituents because of lot-to-lot differences and alteration of component concentrations over time, caused by degradation. The fourth factor is differences in cell culture growth surfaces such as vessel surface treatments, supplier variability, and lot-to-lot variability.
Lastly, there are biological issues with cell lines due to carrying cells in continuous culture for prolonged duration, which raises the risk of phenotypic drift, cross-contamination with other cell types, and contamination of infectious agents.
The results and interpretation of data obtained from downstream assays can be adversely influenced by these controllable factors, which can lead to time loss and wastage of costly reagents. The National Chemical Genomics Assay Guidance Manual version 5.0 offers a good overview of what is needed to set up high-quality assays [2].
Approach and results
A label-free, non-invasive method of monitoring cells periodically in their native environment, within the cell culture incubator, is enabled by using the automated live-cell analysis system, IncuCyte. The resulting data comprises of objective, image-based growth metrics, as shown in Figures 1 and 2.
Additionally, the IncuCyte can monitor many different types of cell culture vessels and simultaneously monitor a number of vessels. There is a change in protein expression, gene expression, and growth characteristics when cells become confluent (1, 2).
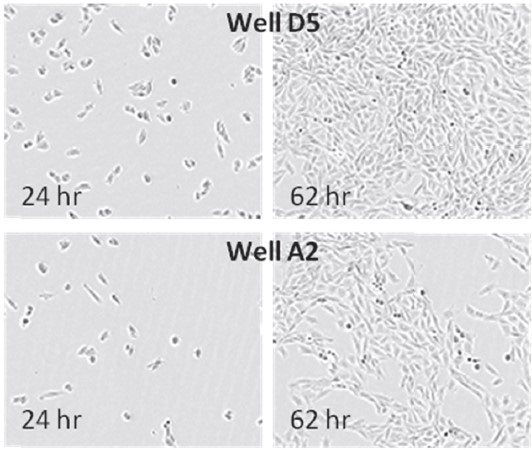
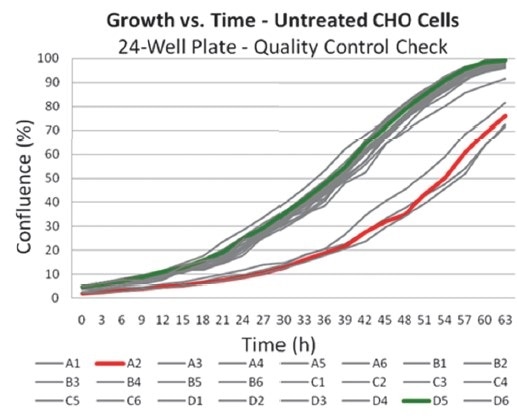
Figures 1 and 2. Images and data derived from IncuCyte illustrating the effect of pipetting errors on growth. Wells in this plate were seeded at the same cell density and then checked prior to an endpoint assay for growth. Initial confluence for four wells were found to be ~50% that of the median initial confluence for the plate; a variable likely induced by pipetting errors. Images from the two wells compared were retrieved from the database for visual inspection and correlation with the confluence data.
During plate seeding, pipetting errors lead to different cell numbers, different growth rates, and heterogeneous cell populations depending on differential expression. This results in increased variability of cell-based assay data. To ensure that experiments are performed at optimized and consistent cell densities, it is essential that confluence is measured prior to utilizing cells for any assay.
A more accurate assessment of the overall cell monolayer and of the spatial variations over various types of vessels is enabled by the exhaustive data captured by IncuCyte (Figures 3 and 4).
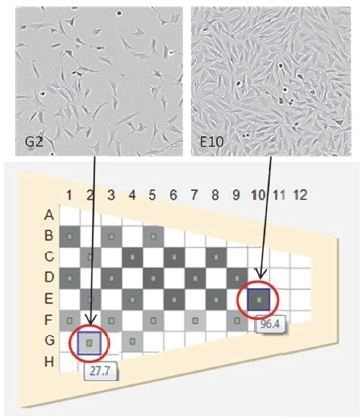
Figures 3 and 4. T-25 flask exhibiting uneven growth, often related to poor culture technique. Shown are two representative images from a single flask exhibiting confluence differences.
By changing the technique, such variations can be removed leading to less assay variation, higher cell yields, and homogeneous cell behavior. Standardization of objective metrics is key to control adverse variables, thus removing human subjectivity and interpretation.
Quality control monitoring with IncuCyte allows automation of the data capture and cell assessment process, unlike the manual monitoring of the cell culture process (Figure 5).
Monitoring of cells around-the-clock at precise, periodic sampling intervals is ensured by using IncuCyte. The obtained data and images can be accessed from any computer on the local network since they are stored in the controller database.
Other than supporting decisions needed for the current culture process, this data is retrievable, months or years later, to compare the characteristics of cell lines and culture growth.
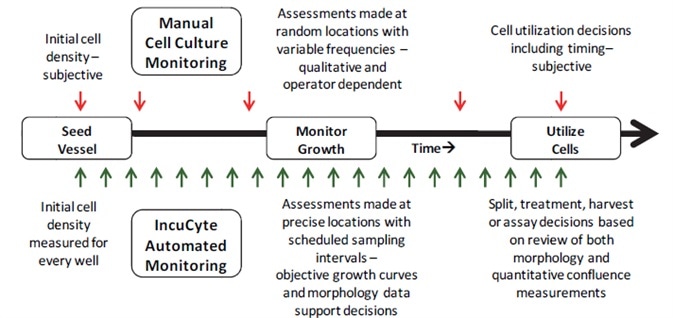 Figure 5. Comparison of cell culture monitoring methods. Vertical arrows represent interaction points with the cell culture for either method. Subjective decisions made during manual cell culture monitoring result in variability for cell-based assays.
Figure 5. Comparison of cell culture monitoring methods. Vertical arrows represent interaction points with the cell culture for either method. Subjective decisions made during manual cell culture monitoring result in variability for cell-based assays.
Conclusion
For years, Sartorius has pioneered the development of assay technologies and cell-based assays, and hence appreciates the issues researchers come across when attempting to control the variables affecting cell-based assays. The experience gained by the company has led to the development of the IncuCyte, which can be employed to improve the probability, quality, and reproducibility of cell-based assays.
IncuCyte allows cell growth to be tracked and quantified over time, and reveals time-dependent and transient phenomena. It is an essential cell culture quality control tool as it provides an objective, quantitative, kinetic, non-invasive analytical method on living cells in their native environment.
Cell-based assay quality and consistency can be improved by employing IncuCyte to document and monitor routine cell culture.
References
1. Hsiao-Wen Su, Shainn-Wei Wang, Fayez K. Ghishan, Pawel R. Kiela, and Ming-Jer Tang (2009) Cell confluency-induced Stat3 activation regulates NHE3 expression by recruiting Sp1 and Sp3 to the proximal NHE3 promoter region during epithelial dome formation. Am J Physiol Cell Physiol.; 296(1): C13-C24.
2. MG Lampugnani, M Corada, P Andriopoulou, S Esser, W Risau and E Dejana (1997) Cell confluence regulates tyrosine phosphorylation of adherens junction components in endothelial cells. Journal of Cell Science, Vol 110, Issue 17 2065-2077.
 Sartorius
Sartorius
Sartorius is a leading international pharmaceutical and laboratory equipment supplier. With our innovative products and services, we are helping our customers across the entire globe to implement their complex and quality-critical biomanufacturing and laboratory processes reliably and economically.
The Group companies are united under the roof of Sartorius AG, which is listed on the Frankfurt Stock Exchange and holds the majority stake in Sartorius Stedim Biotech S.A. Quoted on the Paris Stock Exchange, this subgroup is comprised mainly of the Bioprocess Solutions Division.
Innovative Technologies Enable Medical Progress
A growing number of medications are biopharmaceuticals. These are produced using living cells in complex, lengthy and expensive procedures. The Bioprocess Solutions Division provides the essential products and technologies to accomplish this.
In fact, Sartorius has been pioneering and setting the standards for single-use products that are currently used throughout all biopharmaceutical manufacturing processes.
Making Lab Life Easier
Lab work is complex and demanding: Despite repetitive analytical routines, lab staff must perform each step in a highly concentrated and careful way for accurate results.
The Lab Products and Services Division helps lab personnel excel because its products, such as laboratory balances, pipettes and lab consumables, minimize human error, simplify workflows and reduce physical workloads
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.