Researchers in the UK have demonstrated the efficacy of the Pfizer-BioNTech and AstraZeneca vaccines against severe acute respiratory virus coronavirus 2 (SARS-CoV-2) at preventing death among older individuals with coronavirus disease 2019 (COVID-19).
The analysis of more than 48,000 individuals, aged 70 years and older, found that COVID-19 cases who had been vaccinated with one dose of Pfizer-BioNTech’s BNT162b2 vaccine were at a 44% reduced risk of death, compared with unvaccinated individuals, while those vaccinated with one dose of AstraZeneca’s ChAdOx1 vaccine were at a 55% reduced risk.
The team from Public Health England in London also found that among cases that had received two doses of BNT162b2, the mortality risk was reduced by 69%, compared with unvaccinated individuals.
The researchers say the study is the first to estimate the effectiveness of ChAdOx1 at preventing mortality and that a single dose offered a similar level of protection as one dose of BNT162b2.
“We found that confirmed cases of COVID-19 who had been vaccinated with either a single dose of BNT162b2 or a single dose of ChAdOx1 had a significantly reduced risk of dying compared to unvaccinated cases,” writes Jamie Lopez and colleagues.
However, the second dose of BNT162b2 offered yet further protection highlights the importance of completing the full two-dose regimen adds the team.
A pre-print version of the research paper is available on the medRxiv* severer, while the article undergoes peer review.
Evidence on vaccine efficacy in preventing mortality is limited
Studies of the real-world effectiveness of vaccines against SARS-CoV-2 have reported high levels of protection against both symptomatic COVID-19 and asymptomatic disease.
However, evidence on the effectiveness against the most severe outcome – mortality – is currently limited and has not yet been reported for most vaccines, says Lopez and colleagues.
The UK was the first country to implement a COVID-19 vaccination program. This began with the administration of Pfizer-BioNTech’s BNT162b2 vaccine in December 2020 and was soon followed by the rollout of the Oxford-AstraZeneca’s ChAdOx1 vaccine, starting in March 2021.
Older adults, care home residents, and health and social care workers were the first to be prioritized for vaccination, with subsequent rollout to increasingly younger age groups and clinical risk groups at later stages.
Early on in the vaccination program, policy-makers decided that the interval time between the two vaccine doses should be extended from 3 to 12 weeks, so as to maximize the number of high-risk individuals who could be offered the first dose.
“Early data suggested that a single dose of BNT162b2 was 80-85% effective at preventing mortality in individuals aged over 80 years,” writes Lopez and colleagues. “However, the effectiveness of ChAdOx1 against mortality has not yet been reported.”
What did the researchers do?
In England, a community COVID-19 testing program is available to people reporting symptoms (such as high temperature and continuous cough), care home residents and staff, and those participating in local or national asymptomatic testing.
Lopez and colleagues linked all new symptomatic cases that were positive for SARS-CoV-2 infection by polymerase chain reaction (PCR) to mortality data from National Health Service (NHS) records.
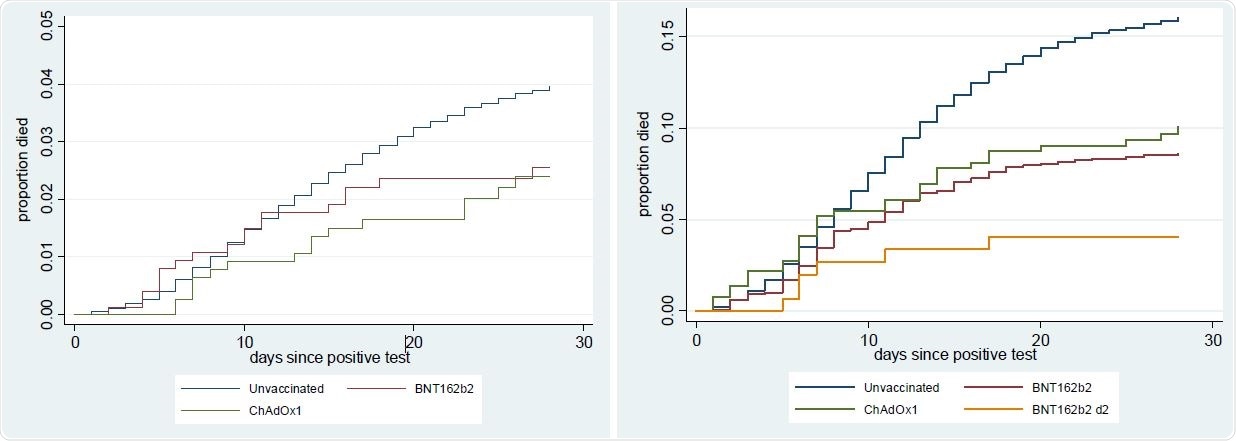
Proportion died by vaccination status (a) 70-79 year olds (b) 80+ year olds
Data on testing and mortality between the 8th of December (when vaccination rollout began) and the 17th of April were extracted, and survival analysis was conducted to estimate the risk of death within 28 days of a positive PCR test.
The mortality risk was assessed among 48,096 individuals aged 70 years and older who had COVID-19. Of these COVID-19 cases, 79.1% were unvaccinated, 12.7% had been vaccinated with BNT162b2, and 8.2% had been vaccinated with ChAdOx1.
What did the study find?
Among individuals aged 80 years and older who had at least 28 days of follow-up data available, 16.1% of unvaccinated individuals died, compared with 9.2% among those vaccinated with one dose of BNT162b2, 11.3% of those vaccinated with one dose of ChAdOx1, and 4.7% of those vaccinated with two doses of BNT162b2.
Among those aged 70-79 years with at least 28 days of follow-up data, 4.0% of unvaccinated individuals died, compared with 2.7% of those who received one dose of BNT162b2, 2.1% of those vaccinated with one dose of ChAdOx1, and 0.0% of those vaccinated with two doses of BNT162b.
None of the individuals had 28 days of follow-up data available after two doses of ChAdOx1 due to the later rollout of this vaccine.
The survival analysis estimated that individuals vaccinated with one dose of BNT162b2 were 44% less likely to die from COVID-19 than unvaccinated individuals, while those vaccinated with one dose of ChAdOx1 were 55% less likely to die and those vaccinated with two doses of BNT162b2 were 69% less likely to die.
What did the authors conclude?
The researchers say the findings provide strong evidence that both the BNT162b2 and ChAdOx1 vaccines offer high levels of protection against COVID-19 mortality after a single dose.
This finding supports prioritizing the first dose in at-risk groups in the context of high disease incidence and vaccine supply or delivery constraints, they add.
“Nevertheless, a second dose of BNT162b2 offers yet further protection against mortality, highlighting the importance of completing the course,” concludes the team.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.