Biopharmaceuticals are made from proteins and other biological molecules. The protein’s aggregation state and molecular weight often define how active it is. The technique of light scattering is becoming increasingly more common as a means of measuring a protein’s molecular weight, aggregates and oligomers. SEC-MALS testing is one of the more well-established light scattering methods for measuring proteins.
The Viscotek SEC-MALS 20 from Malvern Panalytical is a multi-angle light scattering detector that includes 20 detection angles for measuring the absolute molecular weight and oligomeric state of proteins. The instrument can also be used to assess the size and molecular weight of protein aggregates and to analyze natural and synthetic polymers.
The Waters Alliance® system integrated with Waters Empower® software is widely used in the biopharmaceutical industry for protein separations. Sophisticated detectors can be added to most SEC instruments owing to the generic digital and analog connections that can be employed to transfer signals between the devices and their software. However, the software packages are often not compatible.
This article describes how Malvern Panalytical’s OmniSEC software can interact with Waters Empower® SEC system to promote flawless exchange of sample sequences. This, in turn, enables OmniSEC to track the injections carried out by the Waters SEC system and to synchronize data collection. Several proteins were used to show the measurement of protein weight, aggregation state and oligomeric state.
Methods and Materials
First, Malvern Panalytical’s Viscotek SEC-MALS 20 detector was linked to the Waters Alliance® system, which contains an e2414 RI detector, an e2695 pump, an autosampler module and a degasser, as shown in Figure 1. In order to synchronize the injections and data collection, the autosampler trigger signal was connected. The RI detector’s analog signal out was linked to one of the analog inputs on the SEC-MALS 20 instrument. The fluidic connections pass from the column to the SEC-MALS 20 detector and then onto the RI detector. This detector is connected at the end of the line so that the flow cell is protected from any increase in backpressure.

Figure 1. The Viscotek SEC-MALS 20 and Waters Alliance system
Malvern Panalytical’s OmniSEC and Waters Empower® software were installed on the same computer, and the sample set method produced in Empower® software was set to run. The sequence data from Empower® was then loaded by OmniSEC and data collection was started. Following this, the OmniSEC software waits for injections in the Waters Alliance® instrument and obtains information as these injections are performed. The separations were carried out on Malvern Panalytical’s PLS5030 SEC column running PBS at 1mL/min. These silica-based columns are designed for protein separation, with their low particle shedding and high resolution making them ideal for protein light scattering measurements.
Bovine serum albumin (BSA) was run as a molecular weight standard and used to normalize and calibrate the detector response. Test proteins trypsin inhibitor and pepsin were dissolved in the mobile phase. In order to promote adverse aggregation, the trypsin inhibitor was warmed in a microwave for a period of 10 seconds. The pepsin sample was not heated because pepsin is already known to contain a significant amount of aggregates. Next, the samples were injected (100μL) at concentrations of around 2mg/mL and the results assessed using the OmniSEC software.
Results and Discussion
The calibration results of the BSA monomer were within specification and careful measurement of the dimer’s molecular weight showed the results to be accurate. The trypsin inhibitor and pepsin were then measured.
A chromatogram of pepsin containing three peaks is shown in Figure 2. The most well defined peak that eluted at 9.7mL is the monomer and the molecular weight was correctly measured as 35 kDa. The previous eluting peak exhibited a high and polydisperse molecular weight, the result of large aggregates being present in the sample. A less well defined peak eluted when the monomer had a lower molecular weight and was the result of some degraded protein being present. Therefore, this sample shows that the SEC-MALS 20 system linked to the Waters Alliance® system is able to determine the molecular weight of aggregates, monomers, and degradation product (Figure 2).
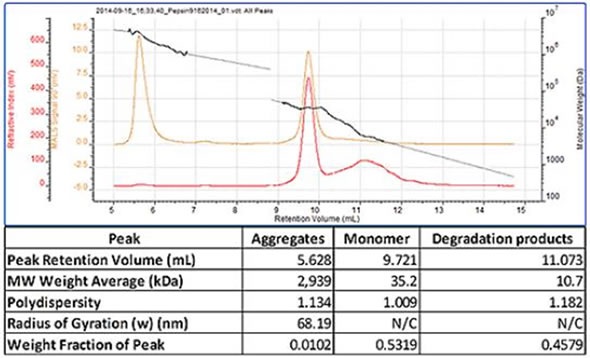
Figure 2. Pepsin chromatogram and table of results.
Shown in figure 3 is a chromatogram of trypsin inhibitor which was heated in a microwave prior to measurement in order to stimulate aggregation. This sample revealed the monomer peak, which had a molecular weight of 27 kDa. Several aggregate peaks were present, which were all examined as a single peak. This had a high and polydisperse molecular weight of 450 kDa, which is typical of such aggregates.
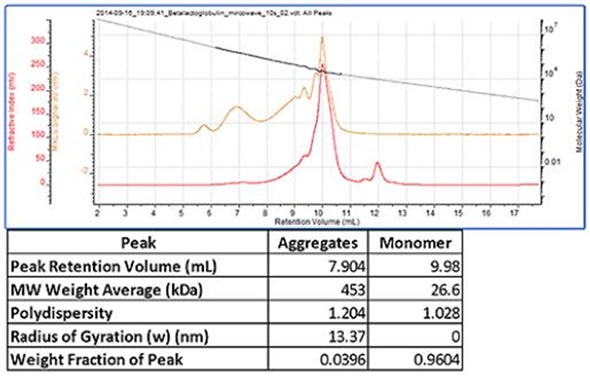
Figure 3. Trypsin inhibitor chromatogram and results
When protein aggregates are above the lower limit of the size measurement (~10 nm), MALS measurements can be used to determine their molecular size. Here, comparisons were made between the sizes of the trypsin inhibitor aggregates and pepsin aggregates. The pepsin aggregates were large, at 68 nm, and had a molecular weight of nearly 3 million kDa, while the trypsin inhibitor aggregates were much smaller, at 13 nm and weighed around 450 kDa. In Figure 4, the variations in the angular dependence graphs can clearly be observed and a single data slice of the two samples of about the same molecular weight (10,000 kDa) is shown. Although the molecular weight is comparable, the pepsin aggregate was 75 nm in size, compared with 15.5 nm for the trypsin inhibitor aggregate.
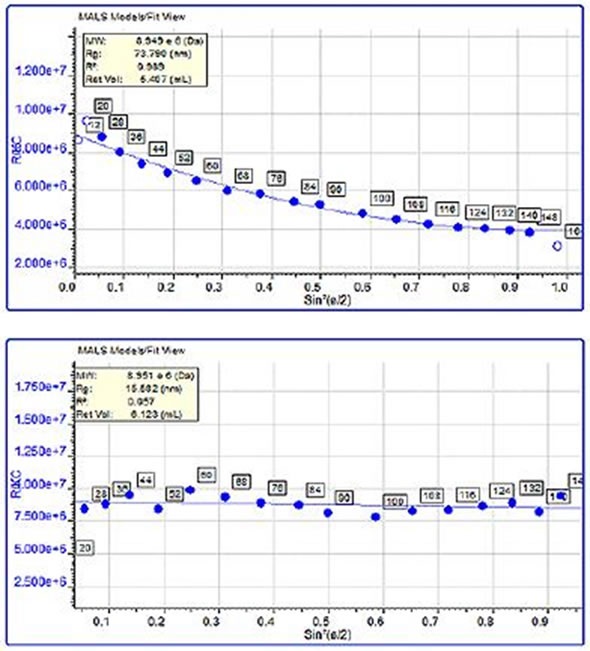
Figure 4. Angular dependence of the scattered light from a single data slice of the pepsin chromatogram along with the measured size. B: Angular dependence of the scattered light from a single data slice of the trypsin inhibitor chromatogram along with the measured size.
Conclusion
This article shows how the Viscotek SEC-MALS 20 multi-angle light scattering instrument can quickly be linked to a Waters Alliance® system to synchronize data collection with Waters Empower® using Malvern Panalytical’s OmniSEC software. Using this setup, the molecular weights of the proteins trypsin inhibitor and pepsin were effectively measured. These measurements show the range of quantifiable molecular weights, from small degradation products in the pepsin sample to large-sized aggregates in the samples. In addition, the sizes of the aggregates in these samples were also determined. The trypsin inhibitor aggregates were relatively small compared with the pepsin aggregates, which were significantly larger, indicating a more open and perhaps more fibrous structure. These kinds of measurements can be easily performed by simply joining the Viscotek detectors to the Waters SEC systems or any other brand of SEC system.
About Malvern Panalytical

Malvern Panalytical provides the materials and biophysical characterization technology and expertise that enable scientists and engineers to understand and control the properties of dispersed systems.
These systems range from proteins and polymers in solution, particle and nanoparticle suspensions and emulsions, through to sprays and aerosols, industrial bulk powders and high concentration slurries.
Used at all stages of research, development and manufacturing, Malvern Panalytical’s materials characterization instruments provide critical information that helps accelerate research and product development, enhance and maintain product quality and optimize process efficiency.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.