Researchers based in Paris, France, have explored the potential use of zebrafish larvae as animal models for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). These larvae are small and cheap, so could prove a suitable medium for rapid mass testing of the disease.
SARS-CoV-2 is the causative agent for the coronavirus 2019 (COVID-19) pandemic that continues to spread globally.
Animal models are frequently used to assess the effects of diseases and treatments, usually either before progressing onto, or to mitigate the need for human trial subjects.
Since SARS-CoV-2 binds to angiotensin-converting enzyme 2 (ACE2) in humans, most animal models used are either Syrian hamsters or mice. SARS-CoV-2 will bind to ACE2 in these species but will do so more readily to human ACE2 (hACE2). Therefore, mice with transplanted hACE2 expression are often ideal candidates. However, this is expensive and difficult to acquire, as are non-human primate models.
Valerio Laghi, an Italian researcher at the Pasteur Institute in Paris, lead a team to explore zebrafish larvae as a potential, cheap, and readily available animal model to use in place of these rare and expensive mammal models.
The study
Zebrafish may at first seem to be an unorthodox choice to test the effects of a mammalian virus on, but there is a surprising number of similar features between these fish and humans. 80% of disease-associated genes in zebrafish and humans are orthologues (convergently homologous genes) and have been used already to model human viruses, such as Herpes, Influenza, and norovirus. Additionally, different animal models may reveal hidden traits of host-virus interactions.
The research team, which also included Dr. Jean-Pierre Levraud, who previously has done a lot of work with zebrafish and viral pathogens, tested both wild-type and type 1 interferon (1 IFN)-crippled zebrafish larvae. IFNs are proteins used by host cells to signal the presence of viruses, thus organisms, where this is crippled, would be potentially hypersusceptible to a number of viral pathogens.
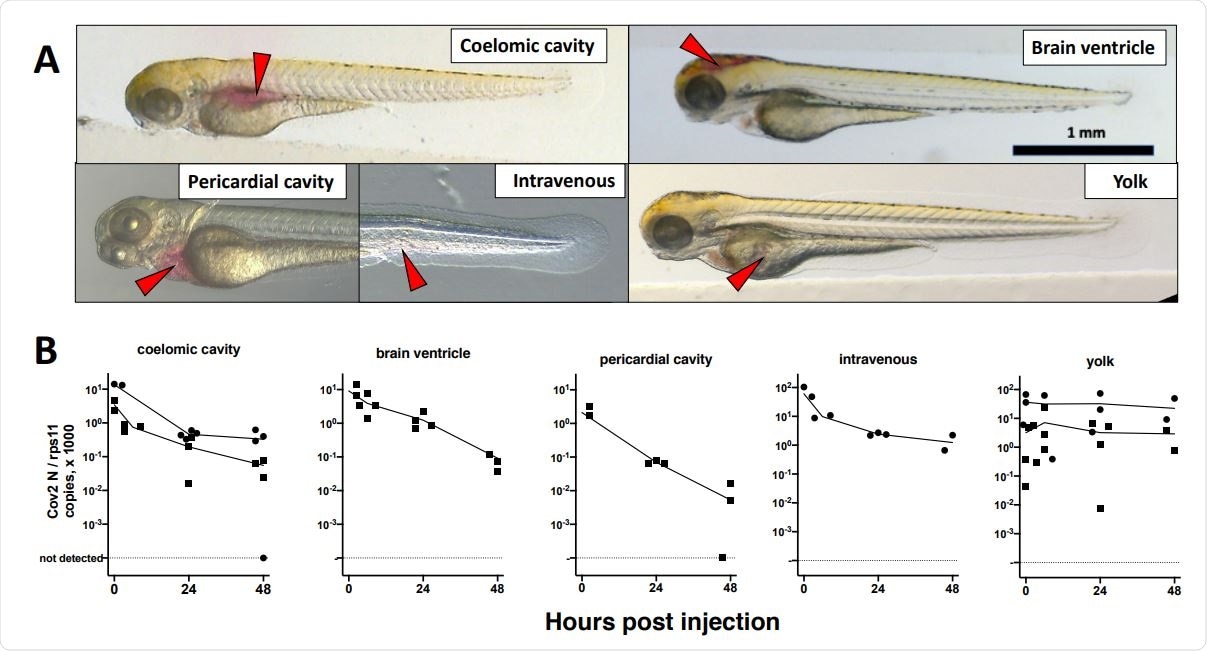
Microinjection of SARS-CoV2 to 3dpf wild-type larvae. A. Illustrations of the targeted sites. Images were taken less than one minute after injection of the phenol red-colored SARS-CoV-2 suspension. Red arrowheads point to the sites of microinjection. B. quantification of polyadenylated N transcripts over time, assessed by qRT-PCR; each symbol is an individual larva.
To establish a mechanism in which zebrafish larvae could be infected, the team initially attempted to inoculate wild-type zebrafish with SARS-CoV-2 by adding viral load to the water of 2 and 4 dpf (days post-fertilization) larvae. After 48 hours post-exposure, however, neither group had been infected. They then attempted to induce infection through microinjections across various sites across 3 dpf larvae, but this also failed infection. Interestingly, RNA injected into the yolk did not degrade, though yolk opacity (a trait seen in larvae infected with some viruses) was not observed.
Finally, the researchers attempted microinjection into the swim bladder and coelomic cavity of 4 dpf larvae. Although no signs of disease were observed, injection into the caudal half of the bladder proved to be successful. Successful RNA replication was observed after a brief period of degradation, thus implying infection had been achieved.
Once a route of infection had been established to induce SARS-CoV-2 replication within the swim bladder, the research team infected type 1 IFN crippled larvae to determine if replication was increased. Mutant zebrafish were bred and then inoculated at 3 or 4 dpf, however, rates of infection did not differ from the wild-type group. This suggests that type 1 IFN was not responsible for lack of infection at other sites in the wild-type zebrafish, instead, they are just not susceptible to SARS-CoV-2.
Furthermore, no inflammatory reaction was observed in the infected larva, and zebrafish with mosaic overexpression of hACE2 was also not sufficient in supplementing infection.
Concluding remarks
The results of this study suggest that zebrafish larvae are not an effective model for SARS-CoV-2. However, asymptomatic infection can be achieved in the swim bladder, most likely due to its evolutionary relatedness to the lungs of tetrapods. The authors posit that the transgenic expression of human ACE2 in the fish could open the potential for this animal model. However, for now, it remains useful in studying the effects of a handful of viral pathogens.
Important notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.