Research has shown that individuals who have diabetes are more prone to develop dementia. Although people are widely aware of diabetes, owing to its common occurrence, they are less aware of prediabetes. Similar to diabetes, prediabetes is also associated with impaired glucose tolerance. However, unlike diabetes, patients with prediabetes show a slight increase in insulin and fasting blood glucose rather than hyperglycemia.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Comorbidities and Neurodegenerative Diseases
Both diabetes and prediabetes are common co-morbidities for the two most common forms of dementia, namely, Alzheimer's disease (AD) and vascular contributions to cognitive impairment and dementia (VCID). Previous studies have also indicated that being obese or comorbid with prediabetes or type-2 diabetes is associated with elevated risks of AD and VCID.
Put simply, AD occurs due to neurodegeneration that negatively affects brain atrophy and causes cognitive impairment. VCID occurs because of a reduction in cerebral blood flow and/or damage to cerebral vessels. It is extremely difficult to differentiate between AD and VCID. Many patients with dementia show multiple pathologies, which is commonly termed mixed dementia (MxD). Despite its high clinical prevalence, a limited number of studies are available on MxD. Scientists believe that MxD could be better understood if the interaction between AD and VCID risk factors were discovered.
Previous research has shown that women are at a higher risk of developing AD and men are more susceptible to VCID. However, this is not true in the case of people with diabetes. Diabetic women are more prone to VCID than diabetic men.
High Fat Diet and Cognitive Disease
Scientists often use a high-fat (HF) diet to induce metabolic disease in rodents. Such diet causes obesity, prediabetes, and it also has an immense effect on the brain. Some of the effects of an HF diet on the brain are sex-dependent. For example, the HF diet impairs adult hippocampal neurogenesis in female but not male mice. Also, another study recently revealed that the HF diet in middle-aged mice resulted in more cognitive deficits in females than males. Prior research revealed that HF diet increased cognitive impairment and AD pathologies like inflammation, brain atrophy, and Aβ load. However, it is still not clear how prediabetes influences different dementia subtypes. Earlier studies documented that HF diet in females increased astrogliosis in the hypothalamus, a brain region that controls metabolic function. Whether these metabolic disturbances induce cognitive impairment and neuropathology in the brain has not yet been investigated.
A New Study
To address these gaps in research, scientists investigated the impact of diet-induced prediabetes and biological sex on cognitive function and neuropathology in mouse models of AD and MxD. This study, which is available on the bioRxiv* preprint server, revealed that diet-induced obesity with prediabetes led to a broader array of cognitive deficits and neuropathology in females compared to males.
In the current research, both male and female 3xTg-AD mice were given a mock (AD model) or unilateral common carotid artery occlusion surgery to induce chronic cerebral hypoperfusion (MxD model). Mice were kept under a controlled or high fat (60% fat) diet for three months before behavior assessment.
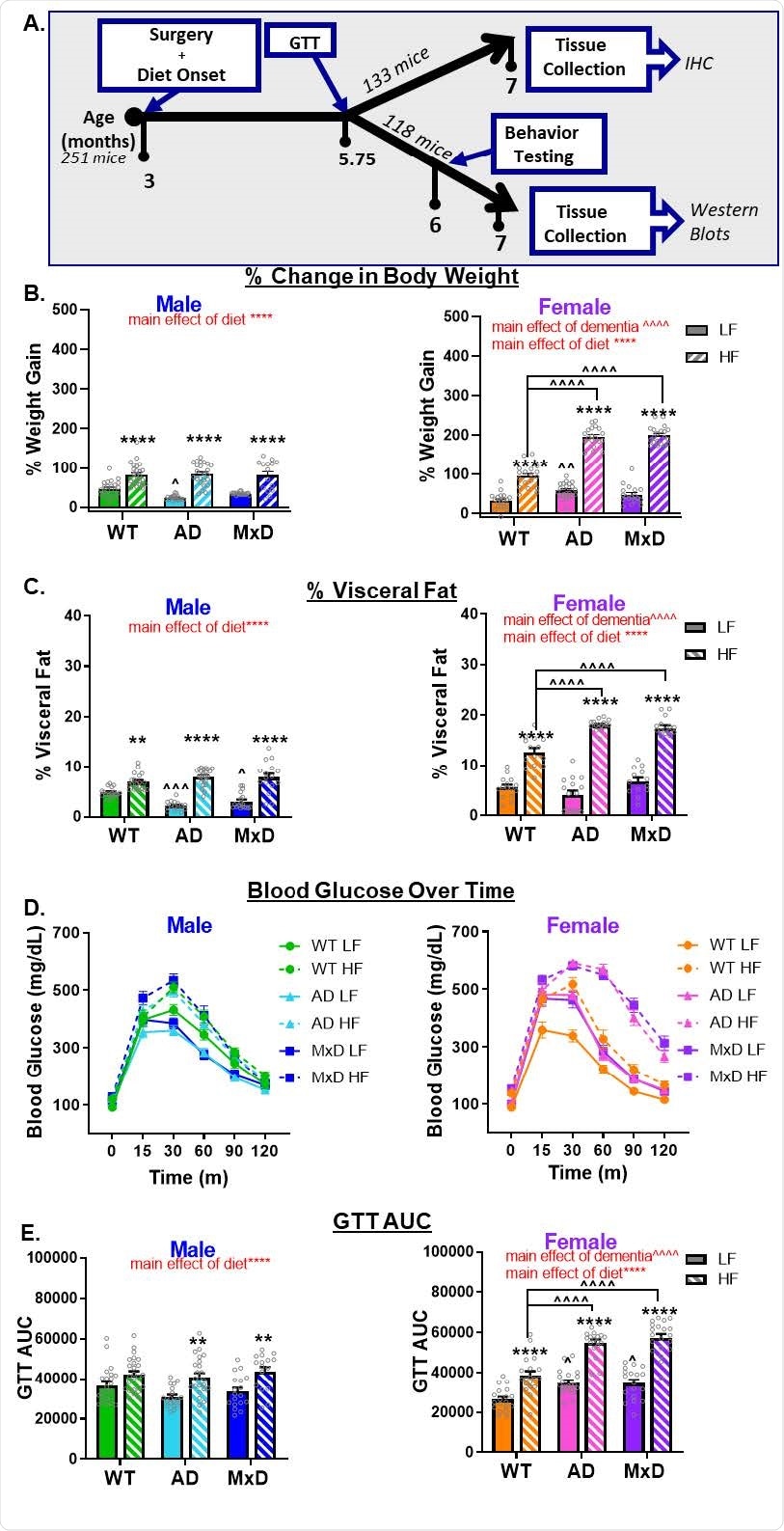
HF diet caused greater metabolic impairment in AD and MxD females compared to males. A) Experimental timeline. GTT (glucose tolerance test). B) Weight gain was assessed by the % change in body weight from the start of the study to the end of the study. C) Visceral adiposity was determined by isolating and weighing the visceral fat pads and normalizing to body weight. D, E) Glucose intolerance was assessed with a GTT following a 16hr fast. D) Glucose clearance was gauged by concentrations of glucose in the blood measured over time (time 0= fasting blood glucose). E) Blood glucose concentration over time was used to calculate area under the curve. We previously reported metabolic data for the Sham WT and Sham AD, but not the MxD, groups in Robison et al (2020) in the Journal of Neuroinflammation (29); licensed under a Creative Commons Attribution 4.0 International License; https://creativecommons.org/licenses/by/4.0/). **p<0.01 effect of diet, ****p<0.0001 effect of diet, ^p<0.05 effect of dementia, ^^^p<0.001 effect of dementia, ^^^^p<0.0001 effect of dementia. Data are presented as mean + SEM (n=13-25/group).
After three months, both the male and female mice gained weight and revealed prediabetic phenotype, i.e., impaired glucose tolerance. Researchers further found that the metabolic consequences of an HF diet were more significant in females than in males as both the groups of female mice with AD or MxD suffered more severely compared to the healthy group of mice (wild type).
The current study showed that both male and female mice, HF-fed AD or MxD, exhibited insufficiencies in spatial memory in the Morris water maze (MWM) study. However, only HF-fed female mice with MxD mice showed reduced spatial learning ability in the MWM. Interestingly, regardless of the diet, female mice with AD or MxD displayed deficiencies in daily activities. Such occurrence was absent in male mice.
Researchers found severe astrogliosis and Aβ pathology among AD and MxD females, compared to males. Additionally, the HF diet resulted in a greater accumulation of amyloid-beta in MxD females compared to MxD males. However, the severe prevalence of prediabetes was correlated with increased hippocampal microgliosis in females, but such a condition was not found in male mice.
Conclusion
In summary, the current study demonstrated that females are more susceptible to the negative metabolic, cognitive, and neuropathological effects of AD, MxD, and an HF diet. The results indicate that prediabetes might influence multiple forms of dementia in women. Differential diets with varied fat compositions or combinations with sugar may have unalike consequences. However, a chronic 60% fat diet causes greater metabolic impairment in AD/MxD females compared to males.
The authors of this study demonstrated the importance of how sex influences the association between risk factors and dementia. Researchers claimed that more work is required for evaluating the overlap of other risk factors, particularly at midlife. These analyses would help develop effective therapeutic approaches for dementia treatment and prevention.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Gannon, J.O. et al. (2021) High fat diet exacerbates cognitive decline in mouse models of Alzheimer's disease and mixed dementia in a sex-dependent manner. bioRxiv 2021.10.05.463111; doi: https://doi.org/10.1101/2021.10.05.463111, https://www.biorxiv.org/content/10.1101/2021.10.05.463111v1
- Peer reviewed and published scientific report.
Gannon, Olivia J., Lisa S. Robison, Abigail E. Salinero, Charly Abi-Ghanem, Febronia M. Mansour, Richard D. Kelly, Alvira Tyagi, Rebekah R. Brawley, Jordan D. Ogg, and Kristen L. Zuloaga. 2022. “High-Fat Diet Exacerbates Cognitive Decline in Mouse Models of Alzheimer’s Disease and Mixed Dementia in a Sex-Dependent Manner.” Journal of Neuroinflammation 19 (1). https://doi.org/10.1186/s12974-022-02466-2. https://jneuroinflammation.biomedcentral.com/articles/10.1186/s12974-022-02466-2.