The evidence for the interconnection between emotional states and intestinal conditions has grown recently. These findings have led researchers to speculate about the association between the brain and the gut – the "gut-brain axis." To understand this functional connection, researchers from Tokyo University of Science used a mouse model for stress-induced psychiatric disorders, called cVSDS, and found that the animals showed symptoms resembling irritable bowel syndrome as a result of repeated emotional stress.
 They found that emotionally-stressed (ES) mice exhibited diarrhea-like symptoms and visceral hyperalgesia without histological changes in the intestinal tract, taking a step closer to understanding the gut-brain interactions underlying IBS. Image credit: Akiyoshi Saitoh
They found that emotionally-stressed (ES) mice exhibited diarrhea-like symptoms and visceral hyperalgesia without histological changes in the intestinal tract, taking a step closer to understanding the gut-brain interactions underlying IBS. Image credit: Akiyoshi Saitoh
Irritable bowel syndrome (IBS) often comes with gastrointestinal symptoms in small and large intestines. IBS has been categorized into four subtypes depending on stool inconsistency; these are IBS with constipation (IBS-C), IBS with diarrhea (IBS-D), mixed (IBS-M), and unclassified IBS. But there is a lack of understanding in the scientific literature regarding the mechanisms and treatments of IBS. One of the reasons for this shortage of knowledge regarding IBS is the lack of useful experimental animal models.
Over the years, studies have suggested a link between emotional states and gut dysfunction, emphasizing on the existence and importance of the so-called ‘gut-brain axis’ in determining our emotional and metabolic wellbeing. Recently, chronic social defeat stress (cSDS) and chronic vicarious social defeat stress (cVSDS) have received acceptance as a model for major depressive disorder (MDD) and post-traumatic stress disorder. Could cVSDS animal models help us understand IBS in detail? To find out, researchers from Tokyo University of Science (TUS) led by Professor Akiyoshi Saitoh from the Faculty of Pharmaceutical Sciences, TUS, used cVSDS mice models. Their objective was to understand the effects of prolonged psychological stress on intestinal conditions. The team found that the psychological stress-induced mice showed higher intestinal transit ratio and visceral pain-related behaviors—hallmarks of IBS. Their findings were published on 6 October 2022 in the journal Frontiers in Neuroscience.
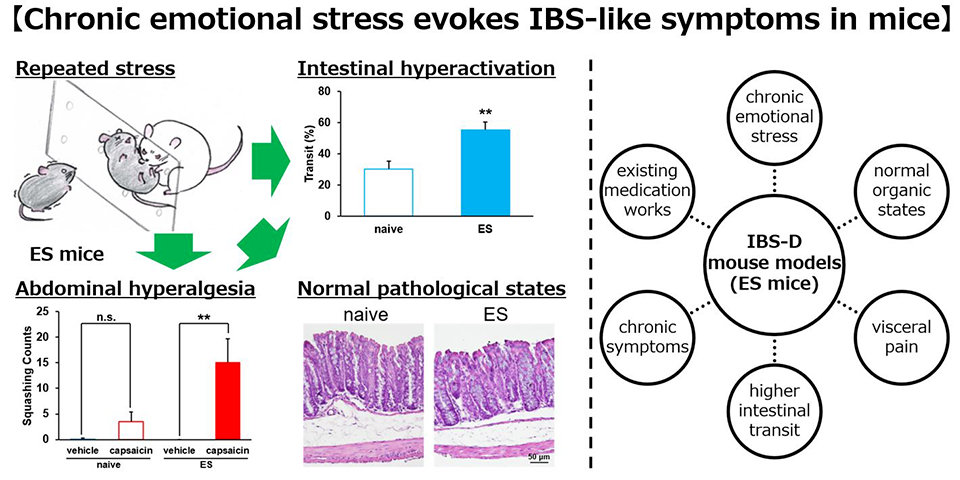
Elaborating on their study, Prof. Saitoh says, "We focused on the cVSDS paradigm and evaluated the impact of emotional stress on intestinal conditions. We further assessed the paradigm's potential as a novel animal model of IBS." In their study, they subjected mice to physical stress or emotional stress, in which the test animals either went through physical aggression or witnessed the aggression for 10 minutes per day for 10 consecutive days. On day 11, social interaction test was conducted to evaluate stress conditions of the test animals. Stress was also estimated through plasma corticosterone quantification, charcoal meal test, and capsaicin-induced hyperalgesia test of the animals. The researchers also evaluated the mice for intestinal permeability, pathology, defecation frequency, and stool content.
They found that the charcoal transit ratio, indicative of passage through the intestine, was significantly elevated in mice that underwent emotional stress compared to mice in the control (naïve) group not exposed to stress. However, the effects were insignificant in mice that underwent physical stress. The defecation frequency and stool water content were also increased in mice subjected to emotional stress. These effects lasted for 1 month after the stress loading. Additionally, there were no significant differences in the pathological status and intestinal permeability between the naïve and emotionally-stressed mice, suggesting no tissue-level changes due to stress.
These results suggest that chronic stress in mice provokes IBS-D-like symptoms, such as chronic intestinal peristaltic exacerbations and abdominal hyperalgesia, without intestinal lesions."
Professor Akiyoshi Saitoh, Faculty of Pharmaceutical Sciences, TUS
Interestingly, the researchers found that the changes in intestinal motility in the test animals were ameliorated when the cVSDS mice were treated with keishikashakuyakuto, a kampo medicine clinically used for the treatment of IBS.
The study highlights the advantage of the cVSDS paradigm over traditional methods in inducing IBS-D-like symptoms through exposure to repeated psychological stress. Speaking about the mechanisms of these effects, Professor Saitoh surmises, "From the aspect of the gut–brain axis, we suspect that the insular cortex plays an important role in determining the phenotype of emotionally-stressed mice.” The insular cortex is a part of the upper central nervous system controlling digestive functions and is involved in the process of coping with psychological stress.
In conclusion, this study demonstrates for the first time that cVSDS-induced psychological stress alone can cause IBS-D-like symptoms in mice. Further research could perhaps rely on cSDS and cVSDS paradigms for elucidating pathophysiological conditions and designing treatments for IBS.
Source:
Journal reference:
Yoshioka, T., et al. (2022) Repeated psychological stress, chronic vicarious social defeat stress, evokes irritable bowel syndrome-like symptoms in mice. Frontiers in Neuroscience. doi.org/10.3389/fnins.2022.993132.