When influenza A viruses and the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infect the human body, innate immune responses in the lung act as the first line of defense against these pathogens, however, little is known about how these responses are controlled locally within the physical microenvironment of the human breathing lung.
Because it is difficult to tease out the regulatory cues in the local environment within a living organ in vivo, and the mechanical cues experienced are absent in conventional culture models, the latest technology of human organs-on-chips is used.
In a recent study, researchers demonstrated a human organ-on-a-chip (Organ Chip) microfluidic model of the lung alveolus (Alveolus Chip) - that recapitulates the human alveolar-capillary interface with an air-liquid interface (ALI) and vascular fluid flow to understand the local triggers in the lung in vitro. They achieved this while allowing independent control over the breathing motions. The results were recently published on the bioRxiv* preprint server.
The researchers found that the cyclic mechanical cues associated with physiological breathing motions (5% strain; 15 deformations/min) sustained moderate levels of production and secretion of an S100A7 protein by alveolar epithelium and endothelium cells. The S100A7 immuno-modulates via RAGE (Receptor for Advanced Glycation End Products)-dependent activation of MAPK and NFκB signaling pathways and generates an innate immune response that protects against viral infection. When breathing motions are absent, the S100A7 levels are lower, and the viral infection is more significant.
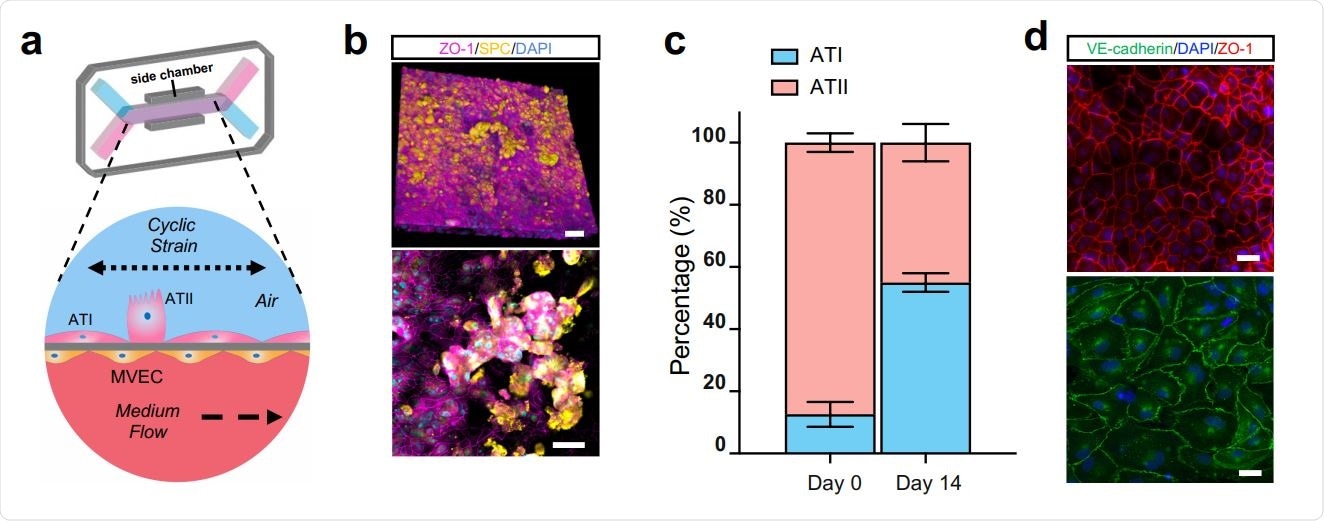
The Human Alveolus Chip. (a) Schematic of Human Alveolus Chip with primary alveolar epithelial type I (ATI) and type II (ATII) cells lining the upper surface of the porous ECM-coated membrane in the air channel with and pulmonary microvascular endothelial cells (MVEC) on the lower surface of the same membrane in the basal vascular channel that is continuously perfused with medium. The entire membrane and adherent alveolar-capillary interface are exposed to physiological cyclic strain by applying cyclic suction to neighboring hollow chambers (grey) within the flexible PDMS microfluidic device. (b) Two magnifications of immunofluorescence micrographs showing the distribution of ZO1-containing tight junctions and ATII cell marker surfactant protein C (SPC) in the epithelium of the Alveolus Chip (bar, 50 μm). (c) Graph showing the percentages of ATI and ATII cells at the time of plating and 14 days after culture on-chip. Data represent mean ± SD; n = 3 biological replicates. (d) Immunofluorescence micrographs showing alveolar epithelial cells (top) and endothelial cells (bottom) within the Alveolus Chip stained for ZO1 and VE-cadherin, respectively (bar, 50 μm).
Further, the researchers also explored whether the RAGE inhibitors can be used to suppress host inflammatory responses during viral infection in the Lung Alveolus Chips. They observed that the effects of mechanical breathing motions on host innate immune immunity in human Lung Alveolus Chips were mediated by modulation of signaling through the S100A7-RAGE pathway.
“Use of human Alveolus Chips can enable us to address questions relating to human lung responses to viral infection and contributions of related breathing motions, which would be impossible in other preclinical, experimental models.”
Human Organs-on-Chips are microfluidic devices lined with human cells - to recreate the natural physiology and the mechanical forces that cells experience. These devices are used to understand the effect of medicines, food, or bacteria on the cells and for drug development and disease modeling.
In this study, the researchers used a Human Alveolus Chip, a microfluidic device containing two parallel channels separated by an extracellular matrix (ECM)-coated porous membrane lined by primary human lung alveolar epithelium cells cultured under an ALI on its upper surface and primary human pulmonary microvascular endothelial cells on the lower surface. A continuous flow of culture medium feeds it through the lumen of the lower vascular channel.
The engineered alveolar-capillary interface is also exposed to cyclic mechanical deformations (5% cyclic strain at 0.25Hz) to mimic physiological breathing motions at tidal volume and the normal respiratory rate of humans via application of cyclic suction to hollow side chambers within the flexible polydimethylsiloxane (PDMS) device.
The infection model the researchers used is influenza H3N2 virus as a pathogen because it is one of the predominant strains in circulation and a leading cause of lower respiratory infection and hospitalization.
“Rapid mutation and reassortment of influenza A viruses also have a high likelihood of leading to the emergence of a novel strain that can cause future pandemics, which constitutes an ongoing threat to public health.”
Importantly, this study also showed that the pharmacological inhibitors of RAGE signaling suppress the inflammation during influenza infection. Thus, the RAGE inhibitors might represent novel adjuvants that may be used in combination with antiviral therapies to suppress life-threatening inflammation of the distal airway that can be triggered by influenza A viruses.
Little is known how inside the lungs, breathing motions and the mechanical forces impact innate immunity and responses to infection. The processes occurring within the lung parenchymal cells and pulmonary microvascular endothelium are unknown.
Because current studies of influenza infection in the distal lung or mouse models do not reflect the anatomy or pathophysiology of the natural human host, the use of human Alveolus Chip enables the researchers to address the human lung responses to viral infection and contributions of related breathing motions.
In this study, the researchers have used the Human Alveolus Chips to mimic the human lung physiology, diseased state, therapeutic effects of a trial drug or any toxicity responses. Using human Organ Chip technology, the researchers enabled the discovery of a novel mechano-immunological control mechanism in which the physiological breathing motions suppress viral infection in human lung alveoli by activating host innate immune responses.
“Our study provides the first evidence indicating that physical forces control innate immunity in nonimmune cells and tissues, specifically in pulmonary alveolar epithelium and microvascular endothelium,” the researchers claim.
The researchers conclude, “in addition to serving as a model system for gaining greater insight into mechanochemical mechanisms that underlie this form of innate immunity, it might serve as a useful preclinical model for identification and optimization of new and more effective antiviral therapeutics for diseases of the distal airway that lead to alveolitis and acute respiratory distress syndrome.”
Journal reference:
- Mechanical control of innate immune responses against viral infection revealed in a human Lung Alveolus Chip, Haiqing Bai, Longlong Si, Amanda Jiang, Chaitra Belgur, Roberto Plebani, Crystal Oh, Melissa Rodas, Atiq Nurani, Sarah Gilpin, Rani K. Powers, Girija Goyal, Rachelle Prantil- Baun, Donald E. Ingber, bioRxiv, 2021.04.26.441498; doi: https://doi.org/10.1101/2021.04.26.441498, https://www.biorxiv.org/content/10.1101/2021.04.26.441498v1