 Study: The early interferon catches the SARS-CoV-2. Image Credit: Maryna Olyak / Shutterstock.com
Study: The early interferon catches the SARS-CoV-2. Image Credit: Maryna Olyak / Shutterstock.com
IFN and antiviral immunity
The main function of IFN is to protect against viral invasions. Typically, type I IFN, which includes IFNα, β, and ω, as well as type III IFN, are associated with antiviral immunity.
IFN-I receptors are present in all somatic cells. Comparatively, IFN-III receptors show tissue-restricted expression and are mostly found in the epithelial cells, which support innate immunity at the viral entry site.
Both IFN-I and IFN-III receptors culminate in the activation of a master transcription factor ISGF3. ISGF3 is composed of the subunits STAT1, STAT2, and IRF9. ISGF3 regulates the induction of IFN-induced genes (ISG), which form a cell-autonomous antiviral state.
IFN and treatment of COVID-19
The success of IFN in treating COVID-19 has been moderate. Scientists believe that in order to increase the success rate of IFN treatment, a detailed understanding of the role of endogenous IFN production during infection is essential.
Previous studies have identified several factors that hinder the effectiveness of IFN. Similar to many coronaviruses, the SARS-CoV-2 genome also expresses factors that inhibit IFN synthesis and response.
Scientists have also revealed that some hosts with genetic disorders inhibit the production of IFN. In some cases, the synthesis of IFN-neutralizing autoantibodies occurs as a result of a genetic anomaly that reduces IFN efficiency.
Prior research has revealed that the proinflammatory character of IFN-I often aggravates the course of advanced disease, especially through the activation of immune cells like monocytes.
It is important to understand the limiting factors imposed by both the host as well as the virus that threaten IFN-related protective mechanisms at the nasopharyngeal mucosae. This is important because SARS-CoV-2 infects the upper respiratory tract of the host and viral replication at this site is associated with its transmission.
The two papers published in the JEM revealed early events of SARS-CoV-2 infection by analyzing both nasal swabs of COVID-19 patients, as well as cellular models of nasopharyngeal epithelium. Upon comparing the results of COVID-19 infected and non-infected healthcare workers, researchers observed the presence of ISG signatures and their dynamics in blood leukocytes in nasal samples collected during the early stage of infection.
Assessment of SARS-CoV-2 viral load
Both studies revealed that the accurate measurement of the expression of selected ISGs could be a potential way to detect early nasopharyngeal viral replication. One of the studies (Lopez et al., 2021) established an ISG score set by the expression of four ISGs; namely, IFI27, IFI44L, RSAD2, and IFIT1. However, in the other study (Cheemarla et al., 2021), the authors stated that measuring the level of chemokine CXCL10 is an adequate method to determine viral loads.
Scientists are highly optimistic about these results and believe that this approach opens a new avenue for the detection of early SARS-CoV-2 infection. The viral load could be determined by analyzing the replication rate of different viral variants.
Mucosal innate immunity to SARS-CoV-2
Both studies provided detailed evidence of mucosal innate immunity to SARS-CoV-2, which could help physicians plan proper treatment strategies. Furthermore, these studies determined disease parameters by studying severely infected COVID-19 patients with autoantibodies to IFN-I.
To this end, when the sera completely neutralized IFN-I (IFNα2 and IFNω), the nasopharyngeal epithelia expressed low ISG scores, despite high viral loads. This might be due to the impaired innate immune response to SARS-CoV-2, which may be an appropriate indicator for the administration of antiviral drugs.
The researchers also revealed that treatment of infected cells with IFNα2 blocks viral replication. Antiviral effects and ISG induction are also blocked in serum-containing anti-IFN autoantibodies.
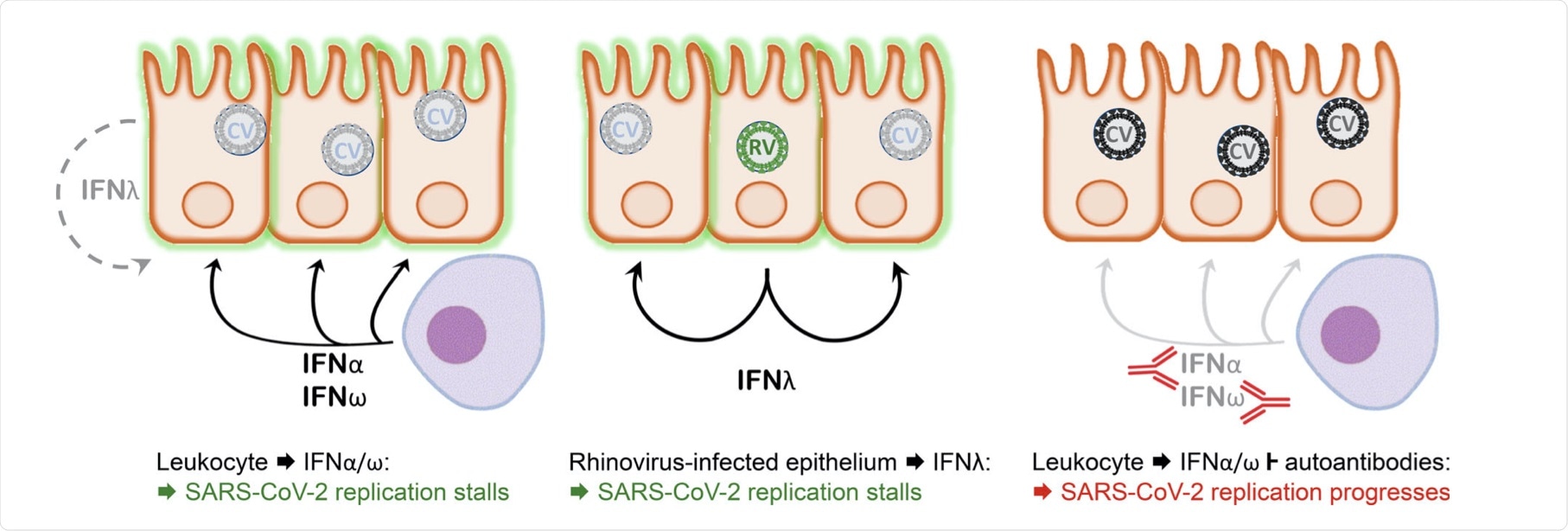 Model depicting the interaction between SARS-CoV-2 and the nasopharyngeal epithelium. Left: In a situation of asymptomatic infection or mild disease, mucosal leukocytes provide the IFN-I IFNα and IFNω for an inhibitory antiviral state. IFNλ production by the infected cells alone is insufficient in this situation. Middle: Infection with an RNA virus such as rhinovirus causes epithelial cells to produce sufficient IFNλ to cause an antiviral state in bystander cells that subsequently lose permissiveness for SARS-CoV-2 replication. Right: As in the left panel, but autoantibodies inhibit the leukocyte-derived IFN. Consequently, the epithelium remains permissive for SARS-CoV-2 replication, allowing for virus spread to the lower respiratory tract and favoring the development of severe pulmonary disease.
Model depicting the interaction between SARS-CoV-2 and the nasopharyngeal epithelium. Left: In a situation of asymptomatic infection or mild disease, mucosal leukocytes provide the IFN-I IFNα and IFNω for an inhibitory antiviral state. IFNλ production by the infected cells alone is insufficient in this situation. Middle: Infection with an RNA virus such as rhinovirus causes epithelial cells to produce sufficient IFNλ to cause an antiviral state in bystander cells that subsequently lose permissiveness for SARS-CoV-2 replication. Right: As in the left panel, but autoantibodies inhibit the leukocyte-derived IFN. Consequently, the epithelium remains permissive for SARS-CoV-2 replication, allowing for virus spread to the lower respiratory tract and favoring the development of severe pulmonary disease.
Conclusion and future research
The current research shows that the IFN response of the mucosae of the upper respiratory tract can inhibit SARS-CoV-2 replication. Scientists agree that the epithelial IFNλ production is not enough to restrict SARS-CoV-2 replication.
Herein, the authors have identified IFNα2 and IFNω as prime mediators of innate antiviral effects. Therefore, they suggest that leukocytes are required for the production of an antiviral state in epithelial cells. The scientists further propose that IFN treatment at an early stage of SARS-CoV-2 infection could inhibit viral spread to the lower respiratory tract which would, in turn, prevent the onset of pulmonary disease.
The JEM studies indicate that both IFNα2 and IFNω possess potential antiviral properties. Although several studies are available that elucidate the role of IFNα, limited studies have been focused on the production of IFNω in humans. More research is therefore required to understand the role of IFNω during SARS-CoV-2 infection and the impact of this cytokine in innate immunity against other respiratory viruses.