
 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Several clinically relevant systemic autoantibodies, such as anti-nuclear antibodies (ANA) and anti-neutrophil cytoplasmic antibodies (ANCA), are prevalent in COVID-19 patients up to one year after recovering from the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
Understanding the significance of these autoantibodies in patients who have recovered from COVID-19 is crucial for understanding the long-term consequences of this fatal viral infection. This will also provide additional insights on the post-COVID-19 risks of immune dysregulation and mechanisms involved in balancing self-tolerance and help manage patients at risk for developing autoimmune diseases.
About the study
In the present study, the researchers performed highly sensitive indirect immunofluorescence (IIF) assays to evaluate the levels of ANA and ANCA. They also performed serum proteomics and human virome-wide serological profiling for comprehensive immunological characterization. In addition, paired analysis of all patients who attended at least one follow-up was conducted to observe changes in ANA and ANCA on an individual level.
The researchers performed human virome-wide serological profiling in 97 acute COVID-19 patients and 18 healthy controls using a total of 87,890 epitopes that consisted of 56-amino acid long and overlapping peptides. ANA-positive and ANA-negative COVID-19 patients were further characterized during acute illness by proteomics, which involved inflammatory markers, cytokine measurements, flow cytometry, clinical history, and routine diagnostic analyses.
The study cohort consisted of 175 confirmed COVID-19 patients followed up to one year after infection, 11 vaccinated individuals, and 41 individuals with no history of SARS-CoV-2 infection as controls.
Study findings
The IIF assay detected ANA titers of 1:320 and above in 41.4% of healthy individuals. This prevalence of ANA positivity was similar to that observed in COVID-19 patients during acute illness, as well as six months and one year after recovery. Interestingly, ANA prevalence was higher during severe COVID-19 and six months post-recovery.
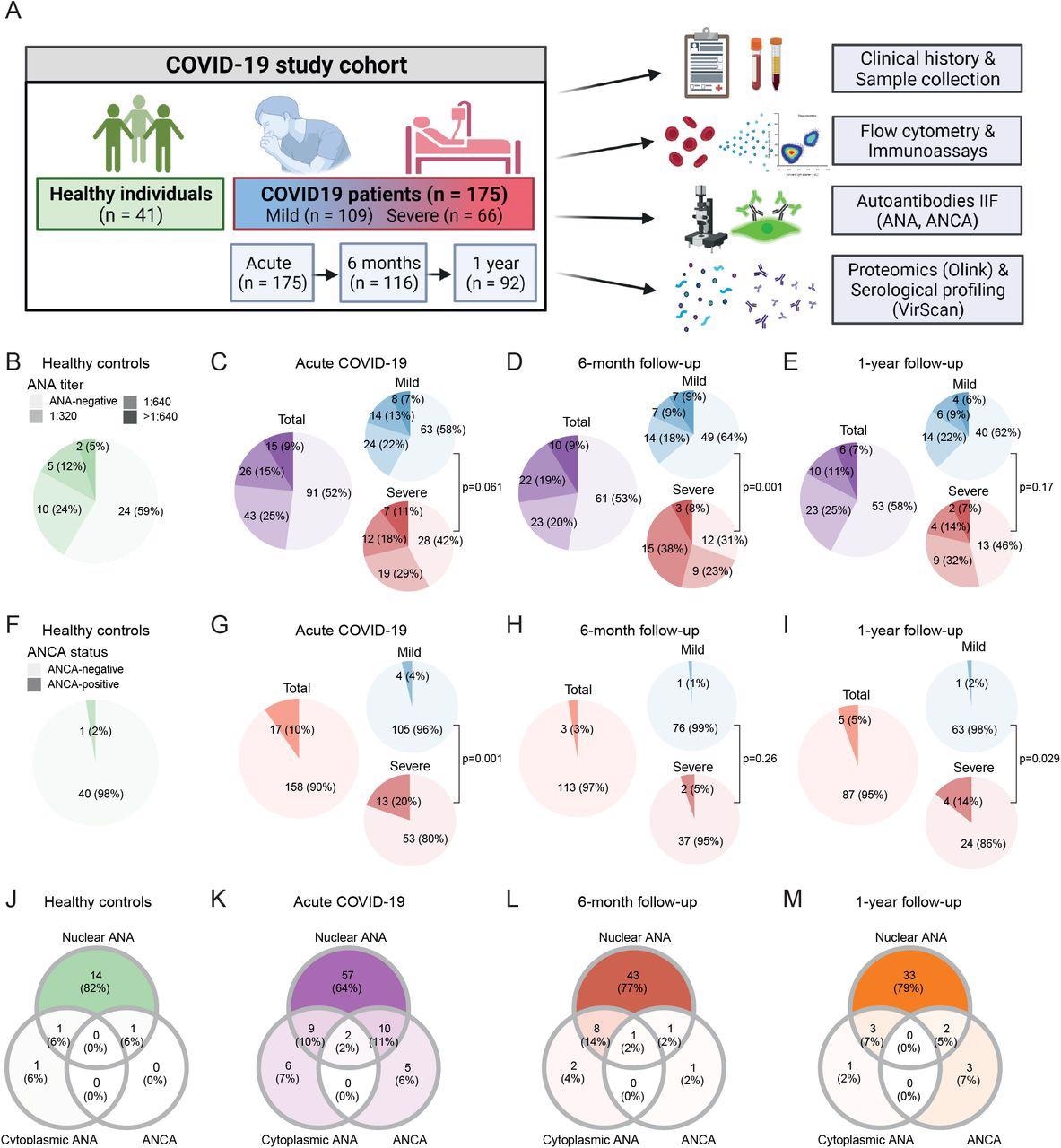
Prevalence of autoantibodies in healthy controls and COVID-19 patients during acute disease and follow-up. (A) Study overview. (B–I) Prevalence of ANA titers (B-E) and ANCA (F-I) in healthy controls (n = 41) and COVID-19 patients during acute disease (n = 175), six months (n = 116) and one year (n = 92) after symptom onset. (J–M) Venn diagrams depicting co-occurrence of nuclear ANA, cytoplasmic ANA and ANCA in healthy individuals (J; n = 17), acute COVID-19 patients (K; n = 89) and COVID-19 patients six months (L; n = 56) or one year (M; n = 42) after SARS-CoV-2 infection that presented with at least one type of autoantibody. P-values indicate comparison of ANA (B-E) and ANCA (F-I) prevalence between mild and severe COVID-19 patients using Fisher’s exact test.
The IIF assay also detected ANCA prevalence in 3.6% mild COVID-19 patients during acute illness as compared to 2.4% healthy individuals. Contrastingly, a significantly higher ANCA prevalence in 19.7% of severe acute COVID-19 patients as compared to healthy and mild COVID-19 subjects. These levels eventually returned to ANCA ranges observed in 5.15% and 14.3% of healthy individuals after six months and one year, respectively.
During acute COVID-19, nuclear ANA, cytoplasmic ANA, or ANCA were detected concurrently in several patients. Comparatively, ANCA prevalence was more frequent in ANA-positive (14.3%) than ANA-negative (5.5%) individuals. Further, in the acute COVID-19 and follow-up period, the cytoplasmic patterns were the most commonly observed ANCA patterns.
Paired analysis results showed that the proportions of patients with isolated ANA positivity during acute illness (14.7%) and follow-up (12.4%) were similar for ANA. However, severely-ill COVID-19 patients (20.5%) showed a trend toward a higher proportion of new ANA development at follow-up visits, compared to 8.2% of patients with mild COVID-19.
A blinded, paired analysis of IIF images showed transient ANA patterns during acute illness. In 17.7% of ANA-positive COVID-19 patients speckled cytoplasmic (AC-19 and 20), nucleolar (AC-8, 9, 10), and mitotic ANA patterns were observed. Contrastingly, only 5.1% of ANA-positive individuals presented an ANA pattern during follow-up similar to that observed during acute disease, thus demonstrating that transient ANA patterns were significantly more prevalent during acute COVID-19.
Of the ten ANCA positive patients during acute COVID-19, eight tested negative for ANCA during follow-up, two remained positive, and one patient exhibited a new positive ANCA result during follow-up. Taken together, the blinded and paired IIF analysis showed transient ANA patterns during acute illness and showed a subset of individuals who showed ANA and atypical ANCA production during the acute phase of COVID-19, which later subsided during follow-up.
Upon investigating the correlation of autoantibodies with specific humoral immune responses to SARS-CoV-2, the researchers observed that the presence of ANA was associated with higher concentrations of S1-specific antibodies in COVID-19 patients during the acute phase, which extended to six months after recovery. Notably, these humoral responses did not sustain up to one year after recovery.
Higher S1-specific IgA and higher IgG titers were also observed in ANCA-positive patients during the acute phase of COVID-19. Together, these findings suggest the prevalence of autoantibodies in conjunction with increased S1-specific humoral responses following acute COVID-19 up to six months after recovery and following SARS-CoV-2 vaccination.
Serological profiling showed distinct differences in COVID-19 patients compared to healthy controls that were particularly pronounced one week after symptom onset. Antibodies directed to all coronavirus species correlated positively with time from symptom onset, thus suggesting the production of cross-reactive antibodies during acute COVID-19.
Further evaluation of antibodies targeting coronaviruses in acute COVID-19 showed a significantly higher proportion of COVID-19 patients who tested positive for a total of 18 coronavirus epitopes as compared to healthy controls, of which 16 were in the spike (S) and two in the nucleoprotein.
Conclusions
The current study highlights that severe COVID-19 decreases self-tolerance by tissue damage and inflammation, thereby leading to the generation of autoantibodies. Furthermore, alterations of the B-cell compartment, including increased naïve and decreased memory B-cells, anas well as differences in total Ig concentrations were observed in patients suffering from post-acute COVID-19 syndrome (PACS).
Furthermore, higher S1-specific antibody titers were observed in autoantibody-positive COVID-19 patients. Similarly, anti-viral humoral responses in such patients were increased during acute COVID-19, although the interrelation remained unclear.
Although the study cohort had few participants with a known autoimmune disease, a higher prevalence of comorbidities in autoantibody-positive patients, including hypertension and heart disease, was observed. In autoantibody-positive COVID-19 patients, an inflammatory signature during acute disease resembled alterations found in severe COVID-19 and changes in inflammation markers, Ig subclasses, and B-cells six months after recovery.
Taken together, the current study demonstrates that autoantibodies in COVID-19 were transient and correlated with increased anti-viral humoral immune responses and a distinct immune signature.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Taeschler, P., Cervia, C., Zurburchen, Y., et al. (2022). Autoantibodies in COVID-19 correlate with anti-viral humoral responses and distinct immune signatures. medRxiv. doi:10.1101/2022.01.08.22268901. https://www.medrxiv.org/content/10.1101/2022.01.08.22268901v1.
- Peer reviewed and published scientific report.
Taeschler, Patrick, Carlo Cervia, Yves Zurbuchen, Sara Hasler, Christian Pou, Ziyang Tan, Sarah Adamo, et al. 2022. “Autoantibodies in COVID-19 Correlate with Antiviral Humoral Responses and Distinct Immune Signatures.” Allergy 77 (8): 2415–30. https://doi.org/10.1111/all.15302. https://onlinelibrary.wiley.com/doi/10.1111/all.15302.