The coronavirus disease (COVID-19) pandemic, caused by SARS-CoV-2, has prompted an unprecedented global quest to develop an effective vaccine. Almost every conceivable vaccine platform has been tried as a solution – including both RNA- and DNA-based vaccines.
Such nucleic acid vaccines can be produced relatively rapidly once a target immunogen sequence is known and can also be modified in line with potential changes in the sequence. However, how to deliver nucleic acids to target cells for immunogen expression is a pertinent technical question.
Thus far, the delivery of RNA vaccines necessitates formulations with lipid nanoparticles or other modalities that protect the genetic material and can convey it across cell membranes. The safety and efficacy of such RNA vaccines are currently being appraised in various COVID vaccine trials.
On the other hand, DNA delivered by needle and syringe may be immunogenic without the use of lipid nanoparticles – even in nonhuman primates. Other techniques (e.g., electroporation or jet injection) can further augment immunogenicity while decreasing dosing requirements.
Recently, a research group led by Dr. Rebecca L. Brocato from the Virology Division of the United States Army Research Institute of Infectious Diseases decided to establish a proof-of-concept to use jet-injected SARS-CoV-2 DNA vaccine by using both wild-type and transiently-immunosuppressed hamsters as animal models.
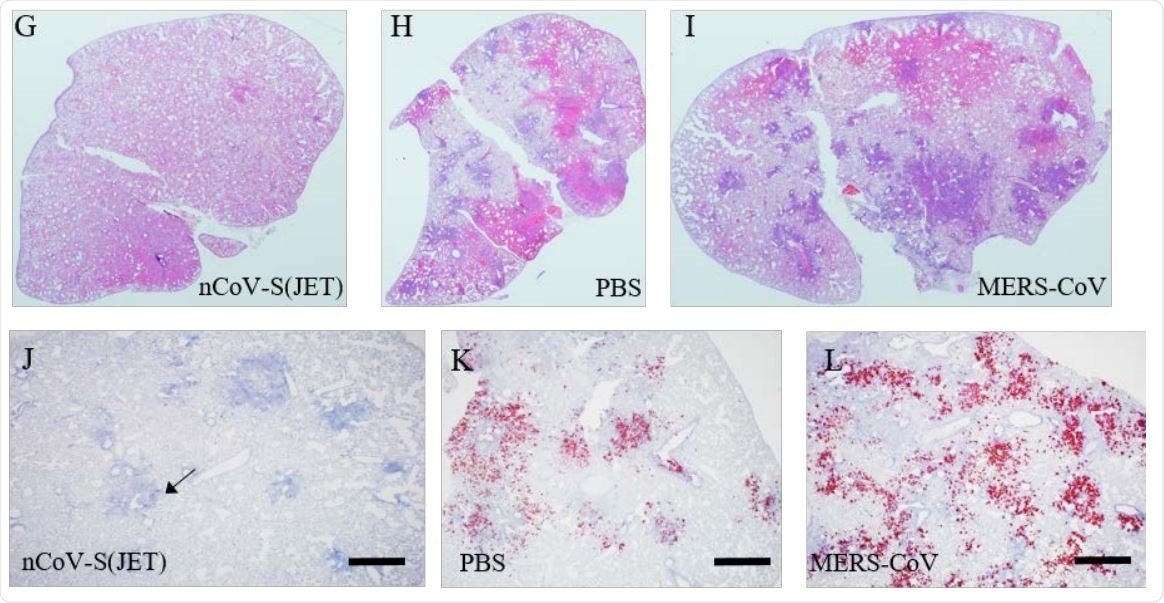
G) nCoV-S(JET) DNA, H) PBS, or I) MERS-CoV vaccinated hamsters where purple indicates areas of consolidation. ISH to detect SARS-CoV-2 genomic RNA in lung sections of J) nCoV-S(JET) DNA, K) PBS, and L) MERSCoV vaccinated hamsters. Rare, positive labeling in nCoV-S(JET) DNA vaccinated hamster lung sections were detected (arrows). Asterisks indicate that results were statistically significant, as follows: *, P<0.05; **, P<0.01; ***, P<0.001; ns, not significant. Scale bars = 400 microns.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Constructing clones and appraising immunogenicity
In this study, scientists have used a jet injection technique to deliver a SARS-CoV-2 spike-based DNA vaccine to Syrian hamsters. Since jet injection technology is not widely available for use in small animals, they have exploited human intradermal jet injections to deliver vaccines intramuscularly to the hamsters.
Their specific SARS-CoV-2 spike-based DNA vaccine, known as nCoV-S(JET), was constructed by cloning a human codon-optimized gene encoding the full-length spike protein into a plasmid vector. Furthermore, the plasmid backbone utilized for this vaccine has been previously used for hantavirus DNA vaccines that are currently in phase 1 and 2 clinical trials.
Primary immunogenicity parameters in the focus of this research were neutralizing antibodies. More specifically, the researchers have measured neutralizing antibodies against the live virus by plaque reduction neutralization test (PRNT) and a non-replicating vesicular stomatitis virus (VSV)-based pseudovirion neutralization assay (PsVNA).
A robust protective effect
In short, the obtained data indicate that the nCoV-S(JET) vaccine-elicited neutralizing antibodies in hamsters and conferred protection in both wild-type and transiently immunosuppressed hamster models.
More specifically, a protective effect was observed even in the SARS-CoV-2 infection model with severe and prolonged disease in immunosuppressed animals before exposure to the virus. Conversely, unprotected animals have lost more than 15% of their weight and still had infectious viral particles in their lungs almost two weeks after exposure.
Moreover, the levels of neutralizing antibody rose substantially after the booster vaccination, with titers comparable or exceeding those found in other DNA vaccines evaluated in nonhuman primates and mice.
Finally, in the case of this novel nCoV-S(JET) DNA vaccine, the normal proliferation pattern of T or B immune cells at the time of exposure was not a prerequisite for achieving the protective effect.
Unveiling potential for further research endeavors
In a nutshell, this study demonstrates how a relatively simple and unmodified full-length DNA vaccine administered by a relatively simple jet injection technique can actually elicit neutralizing antibodies after a single vaccination, but also increase antibody titers significantly after a booster shot and protect against SARS-CoV-2 in two hamster models.
"Our results in the transiently-immunosuppressed hamster model add extra credence to the idea that antibodies are playing an important role in the protection observed," further accentuate study authors in this bioRxiv paper.
This undoubtedly reveals a great potential of this approach to move towards the next stage of preclinical studies and introduces Syrian hamsters as a viable disease model; however, further studies are needed to evaluate these innovative research steps.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources