Sympathetic innervation is crucial to maintain metabolic homeostasis. Advances in neuroanatomy and functional evaluation have deepened our knowledge of the neural network regulating metabolic processes, offering precision in investigating the nervous system. The metabolic regulation by the sympathetic nervous system (SNS) interacts with the immune system, allowing the body to sense and respond to various internal and environmental challenges effectively. The present review highlights the complex neuroimmune relationships affecting the metabolism of the liver, intestine, pancreas, and adipose tissues, potentially revealing novel therapeutic avenues for these interconnected systems.
Sympathetic-immune landscape in the pancreas
The pancreas plays a vital role in the endocrine and exocrine functions of the body. While the nervous system's role in regulating the pancreas is established, increasing evidence suggests additional immune system involvement. The pancreas gets sympathetic innervation from the thoracic and upper lumbar spinal cord segments. The sympathetic control, mediated by norepinephrine (NE), influences blood vessels, pancreatic islets, and exocrine acinar cells. Sympathetic stimulation typically increases blood glucose through elevated glucagon, reduced insulin secretion, and decreased blood flow, while sympathetic inhibition has the opposite effect. Additionally, sensory afferent innervation contributes to the overall activity, ensuring proper pancreatic function and glucose metabolism.
Sympathetic innervation in pancreatic islets influences immune modulation, playing a crucial role in protecting against autoimmune diabetes. In type 1 diabetes (T1D), sympathetic nerve loss is observed, impacting the autoimmune response. Inhibiting sympathetic innervation or α1-adrenergic receptor blockade effectively halts the autoimmune response. Pancreatic islets harbor resident macrophages displaying a pro-inflammatory state, contributing to inflammation and sympathetic nerve damage. Targeting adrenergic receptors on immune cells reduces pro-inflammatory cytokines, preserves β-cell function, and mitigates T1D development in mice.
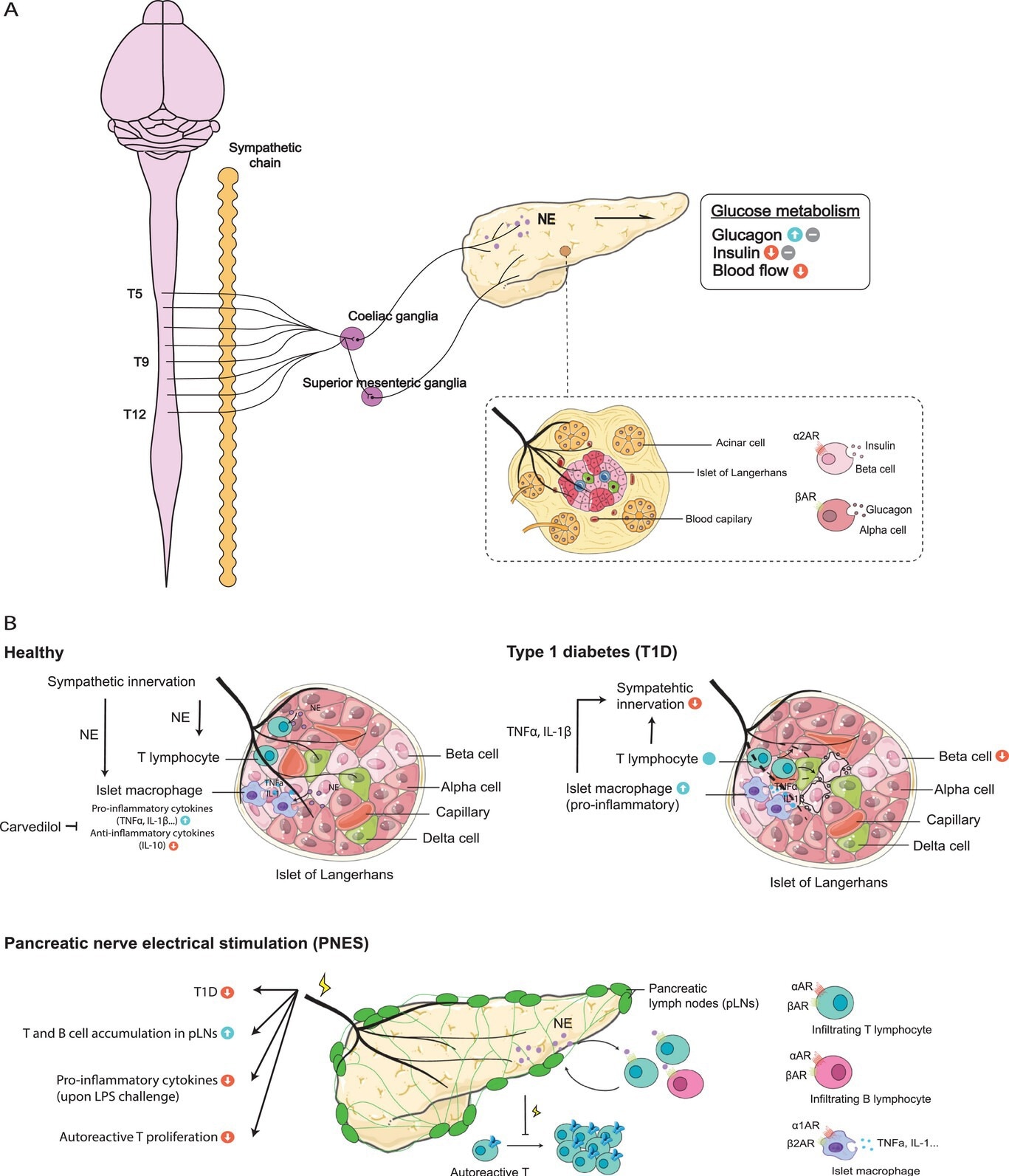
The sympathetic innervation and immune milieu in the pancreas. A) The pancreas receives sympathetic preganglionic fibers from T5-T12 of the spinal cord, while postganglionic fibers primarily originate from the coeliac ganglia and the superior mesenteric ganglia. The sympathetic nerves project to various compartments of the organ, regulating the pancreas’ glucose metabolism. B) The sympathetic-immune crosstalk in healthy and diseased settings of the pancreas. The pancreas harbors resident macrophages and T lymphocytes during steady state, the number of which increase in type 1 diabetes (T1D), leading to a loss of sympathetic innervation and pancreatic β-cell damage. Pancreatic nerve electrical stimulation (PNES) has been reported to reduce the incidence of T1D, inhibit the pro-inflammatory states following LPS stimulation, and prevent autoreactive T cell proliferation in the pancreas lymph nodes (pLNs).
Sympathetic-immune landscape in the liver
The liver is a vital organ regulating hemodynamics, glucose and lipid homeostasis, and immune processes. It receives sympathetic innervation from neurons in the coeliac and superior mesenteric ganglia. Sympathetic activity in the liver influences glucose metabolism, promoting glycogenolysis, hepatic gluconeogenesis, and inhibiting glycogen production. This regulation involves α-adrenergic receptors and neuropeptides. Liver transplant studies suggest a potential role of the autonomic nervous system (ANS) in long-term liver metabolism regulation. In nonalcoholic fatty liver disease (NAFLD), increased sympathetic activity and decreased parasympathetic tone are found to contribute to disease progression.
The liver's immune cells are closely associated with axons of sympathetic neurons. In hepatocellular carcinoma (HCC), sympathetic innervation activates α1-adrenergic receptors on Kupffer cells, promoting HCC development and sustaining inflammation. Sympathetic nerves influence NKT-cell numbers, with NE treatment restoring hepatic NKT-cells and modulating cytokine production. Studies indicate the role of sympathetic innervation in protecting the liver from fulminant hepatitis and promoting regeneration through the ILC1-IL-22 (short for innate lymphoid cells type 1-interleukin 22) axis. The bidirectional relationship between sympathetic nerves and immune cells in the liver requires further investigation for a comprehensive understanding of the underlying mechanisms.
Sympathetic-immune landscape in the intestine
The gastrointestinal (GI) tract is the body's largest immune reservoir, comprised of a variety of cells that are collectively regulated by intricate innervation. During development, axons of sympathetic neurons follow arteries, extending into the gut wall. Sympathetic innervation modulates digestive functions, including secretion, motility, sensation, and epithelial cell proliferation, with specific receptors found in intestinal stem cells and various gut epithelial cells. SNS is found to be involved in various conditions, such as intestinal parasitic infections, inflammatory bowel disease (IBD), and irritable bowel syndrome (IBS).
The neuroimmune interactions of the GI tract influence immune cells like macrophages, lymphocytes, mast cells, dendritic cells, and innate lymphoid cells (ILCs). Sympathetic innervation contributes to distinct macrophage phenotypes in different GI regions, influencing tissue-protective responses and infection outcomes. The neuron-ILC2 units in the intestine highlight a regulatory circuit where adrenergic neurons impact ILC2 activity, which is crucial for immune responses against helminthic infections. Additionally, bacteria possessing functional adrenergic receptors respond to host catecholamines, indicating potential strategies for combating bacterial infections in the gut.
Sympathetic-immune landscape in the adipose tissues
A growing body of evidence suggests that adipose tissue is not merely a passive fat reservoir but a metabolically active endocrine organ contributing to glucose homeostasis, thermogenesis, insulin resistance, and immune responses. Sympathetic innervation is found in both white and brown adipose tissues. Adipose tissue macrophages, eosinophils, ILC2s, and other immune cell subsets respond to sympathetic signals, affecting functions like thermogenesis and inflammation. Disruption in the neuroimmune interaction can compromise nerve integrity and tissue function, emphasizing the importance of preserving neuroimmune balance for optimal metabolic health.
Conclusion
The present review emphasizes the crucial role of sympathetic innervation in orchestrating metabolic processes across various organs, highlighting its impact on immune microenvironments. The potential for novel therapeutic strategies targeting the sympathetic nervous system is recognized to manage conditions such as metabolic disorders, chronic pain, cardiovascular issues, and cancer.
Journal reference:
- The sympathetic-immune milieu in metabolic health and diseases: insights from pancreas, liver, intestine, and adipose tissues. Ren, W., Hua, M., Cao, F., Zeng, W., Advanced Science, 2306128 (2023), DOI: https://doi.org/10.1002/advs.202306128, https://onlinelibrary.wiley.com/doi/10.1002/advs.202306128