Dynamic light scattering (DLS) is a formidable method that is amenable to the measurement of biological samples, especially proteins and biopolymers. One of the quickest growing groups of pharmaceutically active biologics are antibodies.
These extremely adaptable proteins are employed as the main functional component in immunoassays and other fast diagnostics, in vaccine manufacture, and as the main element of a broad range of injectable protein drugs.
In each of these applications it is critical that antibodies stay complete and monomeric. Neglecting to follow these stringent and thorough standards compromises the processability, activity, and shelf stability of antibody-based products.
When DLS is employed to its full potential, aggregation of proteins can be found and the size of monomeric protein may be ascertained to a very high degree of accuracy. To show this theory, aggregation studies performed on pharmaceutical-grade monoclonal antibody (mAb), are compared with results acquired for a common protein, serum albumin (BSA).
Introduction
DLS depends on the principle that freely diffusing material, traveling randomly because of Brownian motion, will generate fast variations in scattered laser light. The timescale of these intensity fluctuations is at the demand of tens of nanoseconds to hundreds of milliseconds. These variations are directly associated with the motion of particles. The signal that develops from the scattered intensity from the laser light is obtained and converted into an autocorrelation function. This is the foundation for measuring a particle size distribution.
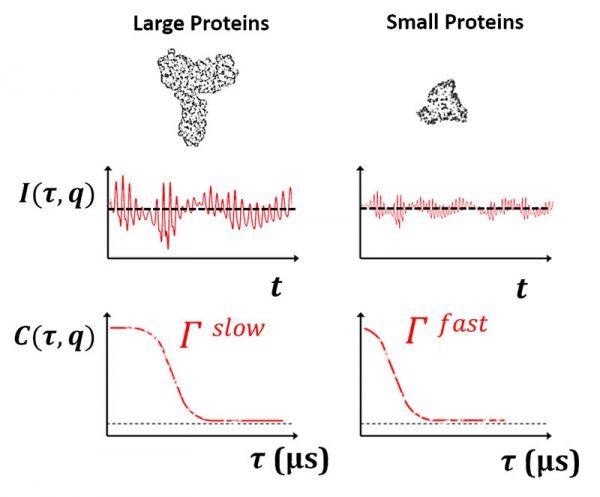
Image Credit: Brookhaven Instrument Corporation
A lot of frequently occurring globular proteins are tiny in hydrodynamic size. Protein monomers are usually <10 nm, with lots of them as small as 1 nm or under. A high-speed correlator is required to measure the size of such quickly diffusing proteins. Protein aggregates can attain large sizes on the order of several hundred nanometers or more with ease. A standard sample comprising partly aggregated protein may extend over 1-2 orders of scale in hydrodynamic size. The difficulty in this instance is selecting the suitable correlator layout to completely resolve these signals. As will be outlined below, the configurability of the correlator is critical to evaluating otherwise challenging heterogeneous biological samples.
It should be noted that the diameter acquired from DLS, frequently known as the hydrodynamic diameter (dh), is inversely proportional to the diffusion coefficient (Dt). The relationship between Dt, measured in DLS, and hydrodynamic size, dh, is inverse, and is provided by the Stokes-Einstein Equation:
Dt = kB T / 3πηdh
Where the Boltzmann constant (kB), temperature (T), and bulk viscosity (η) are all established values. This expression can be simplified to a proportionality:
Dt ∝ 1/dh
The convention in light scattering is to denote small scattering angles as forward-scatter, and large-scattering angles as backscatter. Backscatter, usually referring to angles much greater than 90o, is extremely commonly employed to measure the dimensions of small, monomer-sized proteins. Conversely, forward-scatter, is especially sensitive to larger species and can be employed to identify the presence of even a tiny amount of aggregated protein. For an established scattering angle, θ, and refractive index, n, the scattering vector q is determined from the following expression:
q = 4πn/λo sin(θ/2)
An established correlation function C(τ) is deconvoluted into either a single-exponential, stretched-exponential, or sum of exponentials. The varying elements of this purpose can be divided into a characteristic decay rate, Г, and usually additionally a polydispersity index. This Г is linked to the translational diffusion coefficient (Dt) as follows:
Г = Dt q2
For a standard medium-sized globular protein, decay rates (Г) as small as several hundred s-1 and as big as 50,000 s-1 may be seen, contingent on scattering angle. A configurable correlator layout improves the capacity to measure such a wide variety of decay rates. As displayed below for BSA, Г is subject linearly to q2 for a particle of an established size. In most likely applications, DLS at a single angle can be employed to solve for Dt, and as such to attain the diameter of the highlighted protein. Common DLS configurations, especially benchtop instruments, usually have backscatter, right-angle, and forward-scatter (θ = 173o, 90o, and 15o).
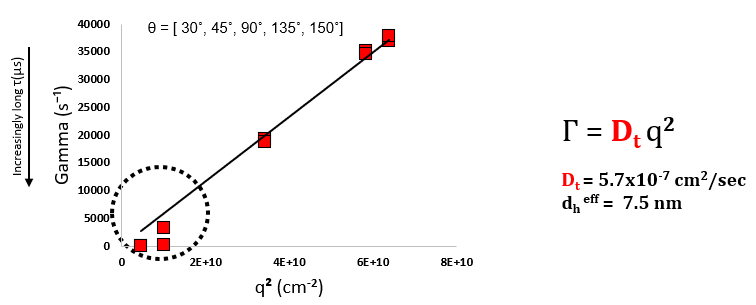
DLS results for serum albumin monomer as a function of scattering angle, θ. Effective dh = 7.5 nm. For this Г vs q2 dependence to be useful, a minimum of 4-5 scattering angles is required. Image Credit: Brookhaven Instrument Corporation
Backscatter can be employed to determine the size of protein monomers. Usually, backscatter angles are helpful for measuring samples where there is a combination of free and aggregated protein. Backscatter angles lessen the inherent intensity bias in the direction of larger particles, which may otherwise obscure scattering from monomeric protein.
Forward-scatter is extremely sensitive to aggregation. In comparison to backscatter angles, extremely low angles are extremely sensitive to big particles particularly those a lot bigger than 1000 nm. Although forward-scatter is extremely sensitive to existence of aggregates, backscatter is more useful for precisely ascertaining the size of any small particles that might coexist with aggregated material.
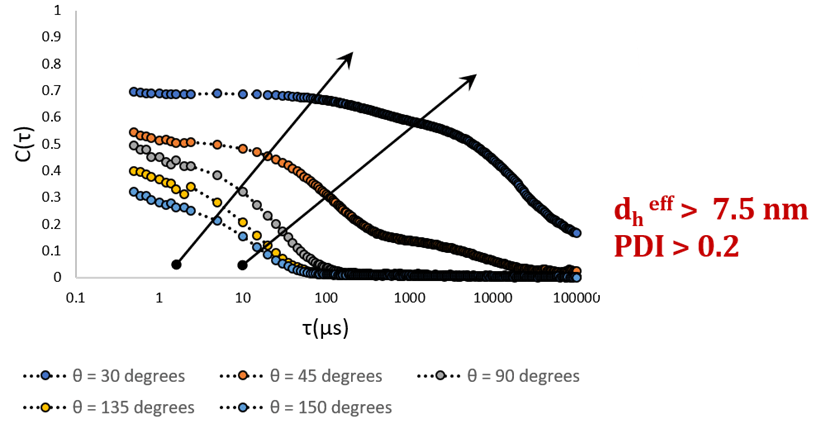
Correlation functions obtained at five separate scattering angles, θ = (30,150°). The existence of a second decay becomes obvious at the two lowest angles. Image Credit: Brookhaven Instrument Corporation
Serum albumin looks monomeric when calculated in backscatter. If a DLS measurement was taken only at backscatter angles, the scattering of BSA could be confused easily for that of pure monomer. The presence of a second decay is just evident at the smallest angles (30o and 45o). A shift in the direction of slower diffusion, or smaller Г, suggests that bigger particles are present. This indicates that big, slow moving particles, conceivably aggregated protein, needs to coexist with monomeric protein.
BSA is a conventional serum protein and is frequently employed as a substitute for more pharmaceutically pertinent proteins like antibodies. This small, globular protein is known as existing in monomeric, dimeric, and sometimes trimeric forms. At physiological pH, an extremely small amount of aggregated BSA may be anticipated. In comparison, antibodies are big, adaptable proteins, with compact subunits, bound together by a lot of, fragile, internal disulfide bonds. Chemically degraded antibodies may either be expected to aggregate, or to generate low molecular weight antibody fragments.
Monomeric mAb may be simply measured employing a single angle. As can be seen below, stable monomeric monoclonal antibody may be measured efficiently at a single scattering angle, θ = 90o. This measurement produces an efficient diameter of approximately 12 nm, and a low, close to zero, polydispersity. The correlation function is best defined as a single exponential decay, implying that the sample is characterized simply and homogeneous, with no aggregate noticed.
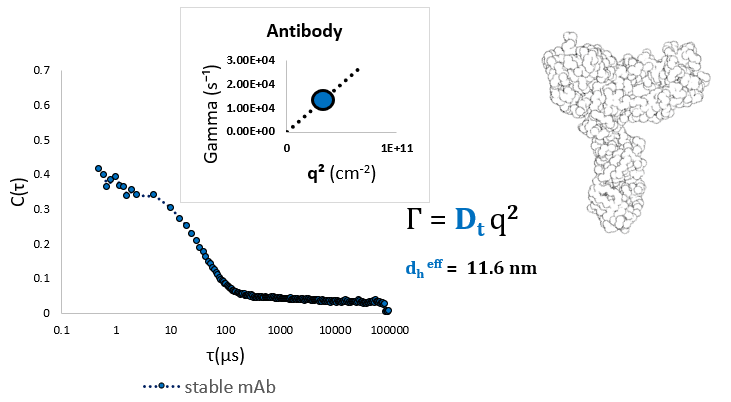
Dilute monoclonal antibody measured at a ninety-degree scattering angle shows a single effective diameter of around 11.6 nm. Image Credit: Brookhaven Instrument Corporation
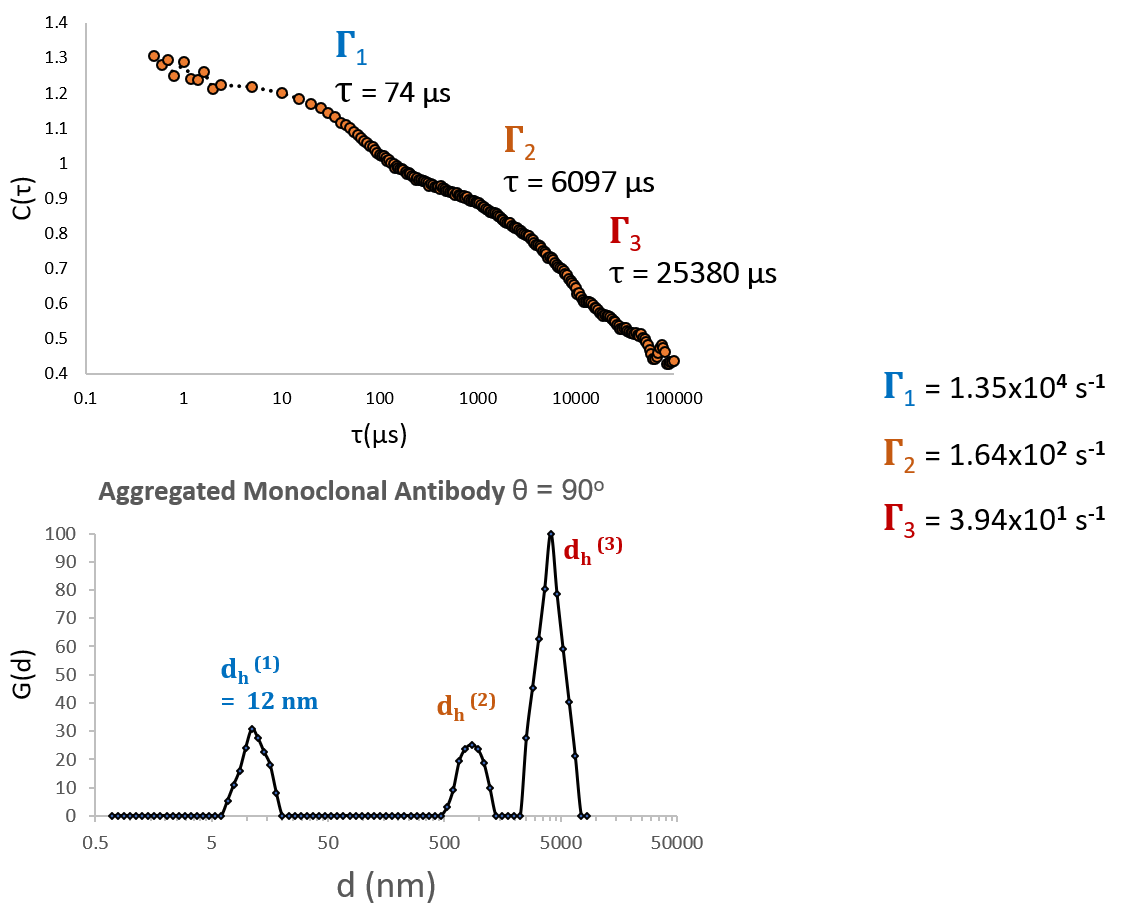
DLS of chemically stressed monoclonal antibody produces a correlation function with 2-3 major components, where the dominant component is aggregated protein. Image Credit: Brookhaven Instrument Corporation
This sample (above) clearly consists of many discrete and well-defined populations, as would be anticipated for a sample consisting of a combination of aggregated and free protein. In comparison, chemically stressed antibody cannot be characterized by a model consisting of a single decay constant, and it cannot be significantly labeled as a continuous distribution of particle sizes. As these Г’s span many orders of magnitude in s-1, they can only be resolved by selecting with caution a correlator layout that encompasses a broad variety of delay times (high, medium and low speed channels).
Free monomeric antibody peaks are entirely resolvable from aggregate peaks when employing a multimodal particle size distribution, as long as appropriate scattering angle (θ ≥ 90o) is employed. It is clear that the selection of a large scattering angle (below) enhances ability to resolve many fast decays.
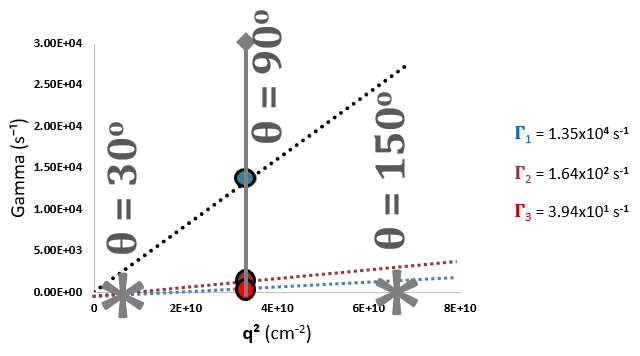
Resolvability of Multimodal Distribution as a Function of Scattering Angle, θ, and Scattering Vector, q2. Image Credit: Brookhaven Instrument Corporation
Considered selection of both correlator layout, consisting of a variety of delay times (τ), and scattering angle permit monomeric mAb to be measured with extreme precision, despite the presence of extremely aggregated protein. The disparity in resolvability of Г1, Г2, Г3, at 15o vs. 155 o should be noted.
Recap
- Choosing an optimal scattering angle is critical to accurately measuring challenging biological samples.
- Constant multi-angle measurements can be helpful, particularly when beginning with undetermined combinations of aggregated and monomeric protein. Despite this, this is frequently needless for reliable, homogenous protein samples.
- It should be noted that forward-scatter denotes very small angles, however backscatter describes high angles. Benchtop instruments frequently have three fixed scattering angles, forward, right-angle, and backscatter.
- Key points:
- Backscatter is helpful for high-precision measurement of small particles or mixtures.
- Low angles are extremely sensitive to bigger particles; this is the reason that forward-scatter is helpful for identifying even small numbers of protein aggregates.
- Forward-scatter angles, while helpful in identifying aggregation, are less useful when it comes to quantifying the sizes of a lot of coexisting species.
Conclusions
Protein aggregates can easily achieve large sizes, on the order of several hundred nm or bigger, which complicate this already difficult measurement. This principle is shown with a pharmaceutically appropriate protein, monoclonal antibody (mAb), examined in both its monomeric and aggregated states. Although it has a heterogeneous nature, considered choice of both correlator layout and scattering angle permits the full resolution the signal from monomeric mAb, despite being in the presence of chemically induced aggregates.

This information has been sourced, reviewed and adapted from materials provided by Brookhaven Instrument Corporation.
For more information on this source, please visit Brookhaven Instrument Corporation.