The current pandemic of COVID-19 has stimulated intensive efforts to develop therapeutic agents against it, whether vaccines or antivirals. A recent paper published on the preprint server bioRxiv* in September 2020 discusses the dynamic interactions of the spike protein glycan shield, which may be of great value in developing therapeutic compounds to fight severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
SARS-CoV-2 is a single-stranded RNA virus with a lipid envelope, studded with spike (S) proteins. These glycoproteins are instrumental in binding the human angiotensin-converting enzyme-2 (ACE2) receptor on the host cell and allowing viral entry into the host cell. Each spike is made up of three identical spike units.
The monomer spike proteins are composed of two subunits, S1 and S2, which are cleaved at the junction, or furin cleavage site, by the TMPRSS2 protease once the virus is bound to the ACE2 receptor. The S1 subunit has an N-terminal domain (NTD) and a receptor-binding domain (RBD) at which the actual binding occurs.
The RBD has a receptor binding motif (RBM) that forms a concave surface. This allows it to make efficient contact with the corresponding convex face of the ACE2 molecule. The protein is also decorated with numerous glycans, including 22 N-glycan and 4 O-glycan sites per monomer.
The RBD of SARS-CoV-2 has different amino acid residues at some critical positions that are thought to account for its much higher affinity for ACE2 compared to the SARS-CoV RBD. Natural mutations at other positions fail to affect the binding affinity, however.
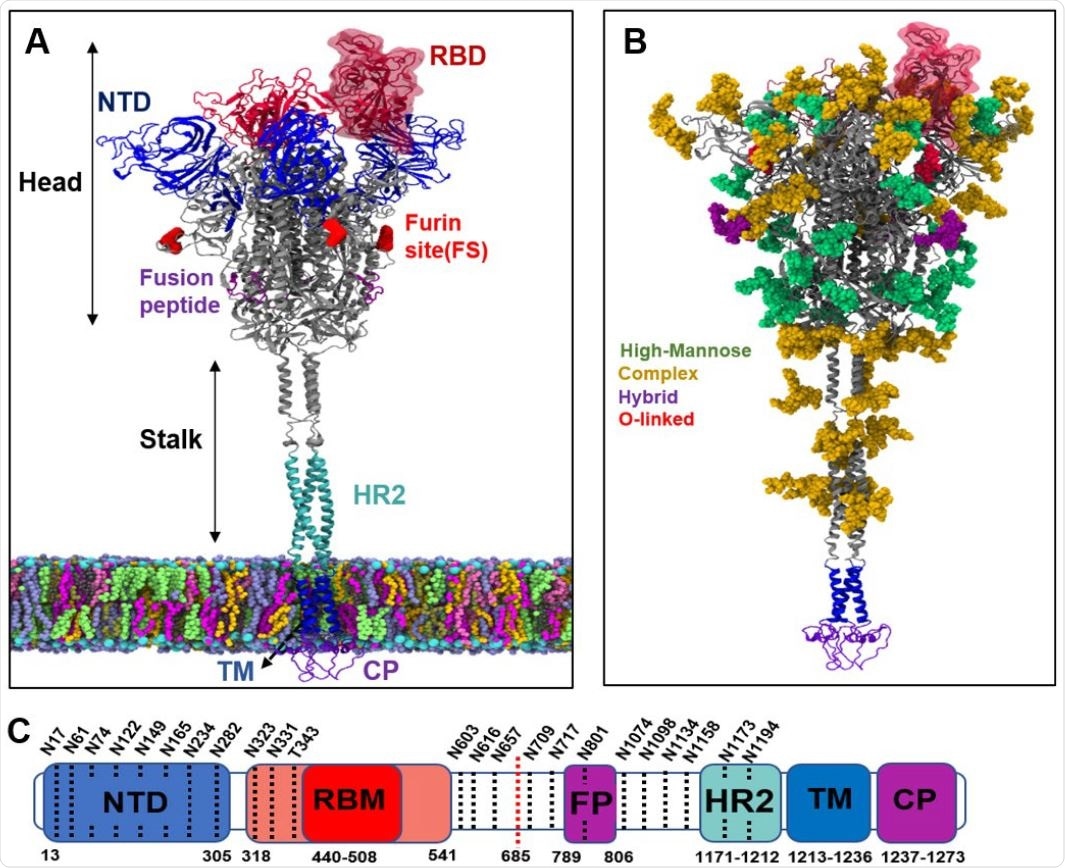
Structure of spike protein and its glycosylation pattern. (a) Different regions of spike protein including N-terminal domain (NTD), receptor binding domain (RBD), Furin cleavage site for cleaving between S1 and S2 subdomains (FS), Fusion peptides (FP), Heptad repeat (HR2), transmembrane (TM) and cytoplasmic (CP) regions. The spike protein is divided into a head and a stalk region. (b) Glycans on the spike protein color-coded based on their types. (c) Sequence of full-length spike protein with domain assignments.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Glycosylation of Proteins
The addition of glycans to proteins is important in their processing and function, including proper folding to attain the right tertiary structure. Glycans also shield the molecule from the host immune system.
The process of N-glycosylation begins with the production of precursor oligosaccharides, to which numerous mannose residues are added via glucosidases, and which is finally trimmed to the right complicated shape in the Golgi apparatus by glucosyltransferases, so that they can act as signaling molecules and fulfil other biological functions.
In protein structure studies, a higher degree of processing indicates that enzymes can gain access to the exposed glycans. Dense glycosylation at any location protects it against processing.
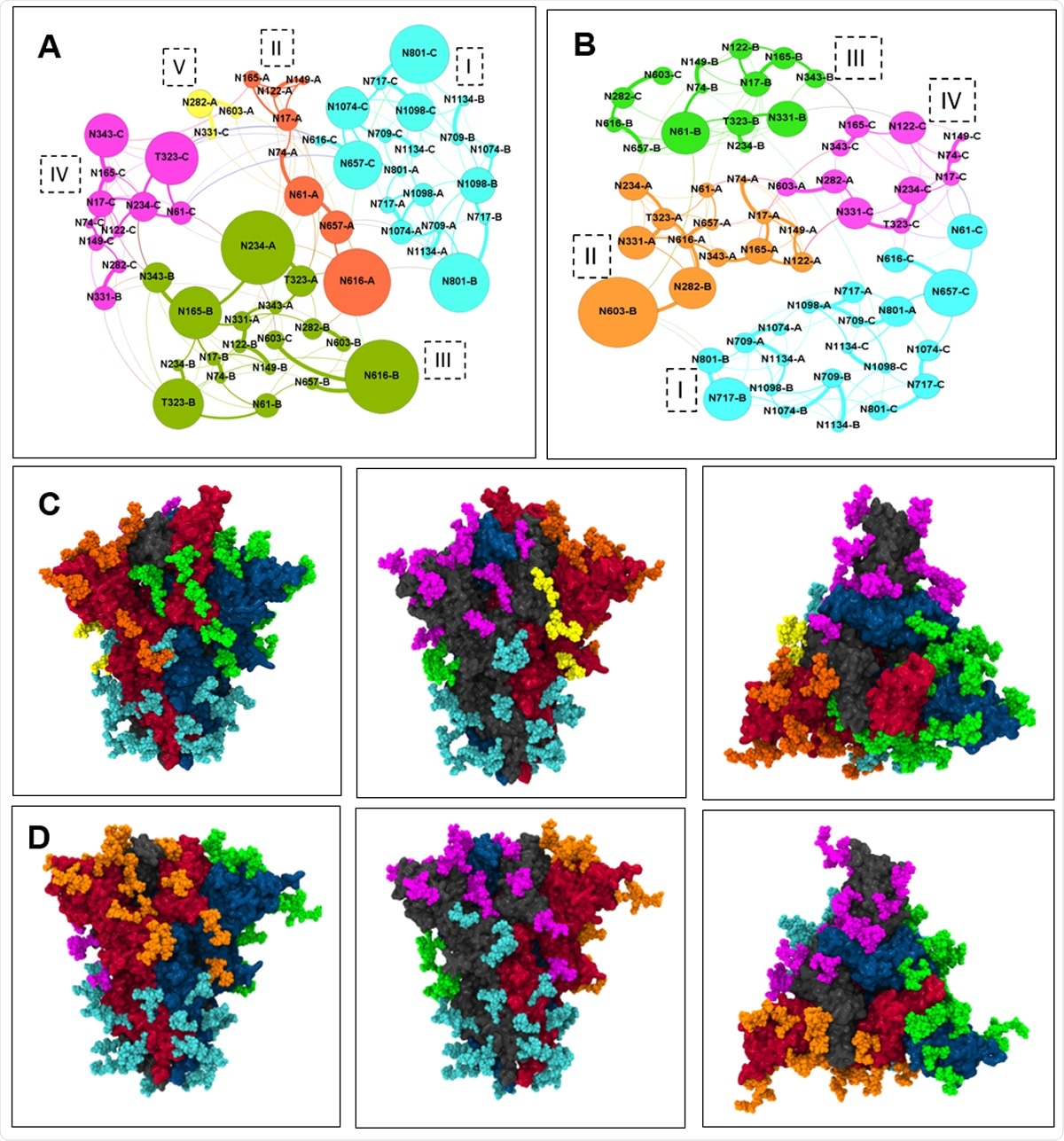
A) microdomains in the open state of spike head with each microdomain color-coded. Glycan are connected through and the thickness of the edge shows the edge weight. B) microdomains in the closed state of spike head. C) microdomains color coded on the spike protein in open state D) microdomains in the closed state of spike head.
MD Simulation Studies
The University of Maryland researchers used molecular dynamics (MD) simulations of the fully glycosylated spike protein in the viral membrane model, in two positions, one with the RBD flap up and the other down (also called the open and closed state respectively). They adopted network analysis and centrality measures, using the graph theory, to identify the structure of the glycan shield, both the interactions of glycans with each other and the spike-antibody binding. They then used a modularity algorithm to identify glycan microdomains where there was a high degree of glycan-glycan interactions and gaps between them where antibodies can attack and neutralize the virus.
Dynamic Motions of The S Protein
The researchers found a bending motion in the stalk region of the spike, in both up and down conformations, with a consequent tilting in the spike head. This may help the virus to rapidly explore the surface of the cell for the ACE2 receptor.
Using RMSF fluctuation analysis, they found that the glycans near the RBD show low values, with two of them being sandwiched between the RBD and NTD of neighboring monomers. Stalk region glycans had high RMSF values, which showed that they shielded the spike heavily in both up and down states.
Scissoring Motion for Receptor Binding
They also found that the RBD undergoes a greater conformational change in the up state, where the NTDs of neighboring chains A and B showed a scissoring motion. The NTD of chain B moved towards the center of the apex as a result, while chain A showed angular motion around the center of the apex, and towards the NTD of chain B. This results in an asymmetrical trimer. This movement is thought by some to be crucial for binding to the receptor and occurs only in the open state.
Another explanation is that this motion is used to hide parts of the spike protein, such as the non-RBM parts of the RBD, when the latter is in the up state.
Incomplete Glycan Occupancy of Spike Protein
There were gaps in the thick glycan shield on the spike head, where antibodies can bind to neutralize the virus. This is unlikely in the stalk region, however. It is important to note that in all the chains, there were glycan-free antibody binding epitopes on the NTD.
In the up state, the RBD of chain A was the least shielded region, with no glycans at all. Some non-RBM regions on the RBD are also glycan-free epitopes.
Among all the chains, the NTD had high SASA, with the highest being in the RBM and NTD of chain A in the closed state, because of the conformational change toward the open state.
The researchers sum up, “Epitopes on RBM, RBD and NTD of spike protein show high SASA for antibodies to bind and neutralize the spike.”
Network Analysis Identifies Essential Glycans
The researchers then used network analysis to examine the glycan shield of the spike protein in both states to pinpoint the most important shielding glycans. They found that stalk glycans showed strong connections to each other to form a strong shield, while on the spike head, particularly at the apex, the glycans, mostly complex glycans, were sparsely connected and appeared to be poorly processed.
The microdomains are regions of high glycan-glycan interactions, and there are fewer edges (and connections) between the microdomains, but a larger number of edges between glycans in the same microdomain. Thus, the region between the microdomains is most susceptible to antibody binding.
The glycans in the lower head are all part of the same microdomain, whether in the up or down state, showing that this part is always well-shielded by the glycans.
They identified two glycans connecting the lower head and the upper head glycans, that appeared to be of central importance in shielding this region. The head glycans show significant separation from the stalk glycans and appear to be more open to binding in the up conformation.
Most glycans in the region of the lower head and upper stalk domain have a high mannose content and are less processed, showing that they are resistant to neutralization by enzymes that process glycans. In fact, no epitopes have been found in this region so far.
Glycans on the head region behaved differently in the up and down conformations, and two showed a high connectedness. One of these (N234A) was found to be inserted in the space left by RBD in the open state and is able to interact with chain B glycans as the NTD of the neighboring chains A and B show their scissoring motion.
The conformational change in the RBD compacts other glycans in the middle head region. The glycan N234A and N165B are essential to stabilize the RBD-up conformation in the open state, as shown by earlier researchers. When these were replaced by alanine, the open RBD conformation became unstable.
These two glycans have a high shielding effect as they interact with each other and other glycans nearby on the RBD of chain A and the NTD of chain B. The spike protein in the open state is more open to attack by antibodies, as shown by the fact that there are five microdomains at the apex in the RBD-up state and only four in the RBD-down state. There are also three apex microdomains of glycans in the closed state, but four in the open state.
Both single-particle cryo-EM and negative stain electron microscopy demonstrated that the ratio of the open and closed state is 1:1. If the N234 glycan is deleted, the ratio shifts to 1:4 in favor of the closed state, while the deletion of N165 produced a 2:1 ratio favoring the open state.
Antibody overlap analysis
The study explored binding pf glycan microdomains by spike antibodies of three types, namely, RBM-binder, RBD-binder (binding to RBD away from RBM), and NTD-binder, in order to compare them with the binding to known epitopes.
They found that the least clashes occurred between the RBM-binder antibody in the open state and the microdomains and the highest with the RBD-binder antibody of chain A. The RBM of chain A had the highest solvent exposed area of all the spike epitopes, showing its suitability as a design template for antibodies to the spike protein.
NTD-binder antibodies also bind to epitopes on the NTD, with a high number of clashes with specific glycans on the chain that they bind. This understanding of the glycan shield will help guide the development of therapeutic molecules against SARS-CoV-2.
Lastly, the researchers comment, “The glycans on the surface of spike protein exert a collective behavior, which is an important property that needs to be considered in the context of vaccine and antibody design.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources