Human HEPATOPAC® cultures can be used for up to four weeks in metabolic stability, met ID, and toxicology applications.

Image Credit: BioIVT
Details
Hepatopac® cultures-human
Human HEPATOPAC® cultures are generated from well-characterized donors. Plates can be adjusted to include a single donor, a pooled donor, or numerous donors in separate wells on the same plate.
The cultures are provided with all required media and a manual for usage instructions. You can start dosing just two days after receiving the kit.
Validation
Human HEPATOPAC cultures remain viable and highly functional for up to four weeks. These cultures exhibit the ability to secrete albumin, synthesize urea, maintain functional bile canaliculi, and process compounds through active Phase I and Phase II drug metabolism enzymes.
The following data demonstrates the morphology, liver function, and metabolizing enzyme activity that is expected from human HEPATOPAC cultures.
Morphology
Human HEPATOPAC cultures were developed and sustained in a laboratory setting for a period exceeding four weeks. Throughout this period, there were no significant changes to the morphology of micropatterned cultures and the polygonal shape was maintained. The movement of a fluorescent dye through MRP-2 evidenced the strong connectivity of bile canaliculi among the hepatocytes.
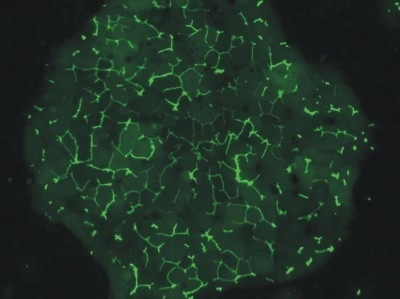
Image Credit: BioIVT
Liver function
The chart below offers a comparison of albumin secretion levels between HEPATOPAC culture and hepatocytes in suspension, highlighting the prolonged viability of the HEPATOPAC culture system.
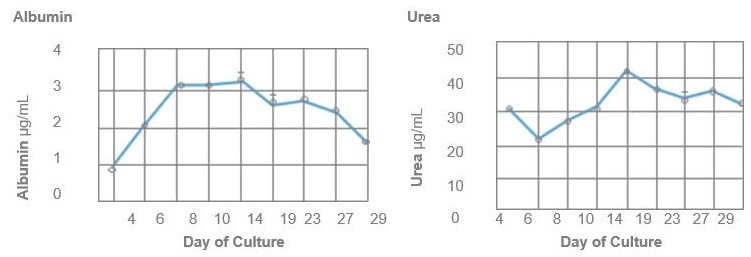
Image Credit: BioIVT
Metabolizing enzyme activity
In human HEPATOPAC cultures, activities of various CYP and Phase 2 enzymes were observed, with metabolites being analyzed through mass spectrometry. The following graphs demonstrate that HEPATOPAC co-cultures sustain their metabolic functions over an extended period.
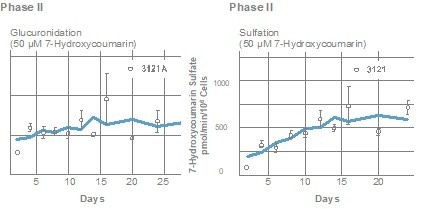
Image Credit: BioIVT
Quality
Specifications
This material should be handled as if it can transmit infectious pathogens. BioIVT encourages users to take universal precautions.
No test method can offer complete certainty that the Hepatitis B virus, Hepatitis C virus, Human Immunodeficiency Virus, or other infectious agents are not present. As a result, all blood products provided by BioIVT should be treated at Bio-safety Level 2 as suggested by the CDC/NIH document “Biosafety in Microbiological and Biomedical Laboratories, from Potentially Infectious Human Serum or Blood Specimens.”
This product is intended solely for research and/or manufacturing uses and must not be utilized in humans or animals. Any further processing leading to a final product containing live leukocytes is strictly forbidden. It is designed for in vitro applications only. Users bear full responsibility for its use and disposal, complying with all applicable regulations.