Chlorine chemistry plays a key role in the manufacturing of thousands of products we depend upon to remain healthy, from disinfecting our public transportation systems and hospitals to keeping our drinking water safe.
Chlorine (Cl2) makes our world a safer place, whether it’s utilized in manufacturing processes or in a final product. Hygiene essentials are in such high demand because of the COVID-19 crisis that there are now critical supply shortfalls for bleach and bleach-based wipe disinfectants.
The U.S. Food and Drug Administration (FDA) released guidance for retail stores, restaurants, and food services during the pandemic to safeguard workers and consumers,1 underscoring the importance of sanitizer and disinfectants production,
The EPA's Emerging Pathogen Policy states that when used as directed on hard, nonporous surfaces bleach products can combat COVID-19.2 Chlorine is the most vital ingredient to produce bleach and bleach related products, but manufacturers need analysis systems that are resistant to metal corrosion due to chlorine’s corrosive nature while supplying speed and sensitivity.
Overview
PerkinElmer’s Chlorine Analyzer is compliant, measures light gases, and outperforms the scope of ASTM E1746 “Standard Test Method for Sampling and Analysis of Liquid Chlorine for Gaseous Impurities.”
The solution utilizes proprietary columns to protect the system from corrosive gases like Hydrochloric Acid (HCl) and Cl2, both of which get backflushed to vent for long-lasting day to day analytical requirements.
It is constructed by utilizing chlorine resistant material for valves, tubing, and all surfaces which are exposed to the sample. The system is not damaged by wet samples and this material enables the analysis of corrosive samples without damage to the analyzer. Two analytical channels are included on the Chlorine Analyzer.
To analyze full range hydrogen, the first channel utilizes nitrogen as a carrier gas. Helium can also be analyzed as nitrogen is the carrier. The second channel is usually referred to as a "light gases" channel, which is employed to analyze methane, carbon monoxide oxygen/argon, nitrogen, and carbon dioxide in chlorine samples.
A crucial feature of the Chlorine Analyzer is the utilization of proprietary packing materials in the first column in both channels. Classic packing materials that are utilized in hydrogen channels and light gases will not retard chlorine long enough to keep it out of the analysis.
The special columns backflush the chlorine to extend the analyzer life cycle whilst still permitting the components of interest to be analyzed.

Figure 1. Chlorine Analyzer. Image Credit: PerkinElmer
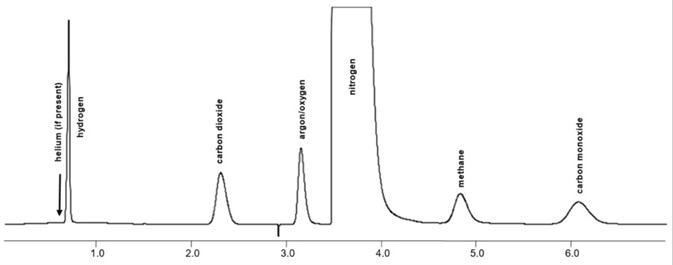
Figure 2. Chromatogram is showing analyzed chlorine contaminates by TCD/TCD. Image Credit: PerkinElmer
Chlorine analyzer step by step analysis description
This analyzer has two analytical channels. It includes three valves, two TCDs, and five packed columns; chlorine resistant valves, tubing and columns allow the analyzer to sample corrosive materials.
The flow paths of the two channels immediately after injection are shown in Figure 3. Until hydrogen fully elutes to the hydrogen channel TCD, the analyzer stays in this configuration. The hydrogen channel is backflushed after hydrogen has fully eluted.
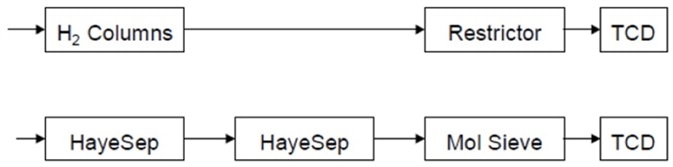
Figure 3. Flow path after injection of sample into both channels. Image Credit: PerkinElmer
As shown in Figure 4, this action protects the molecular sieve column from poisoning by transporting all of the components which are heavier to vent than hydrogen to, including chlorine. This channel is in the ready position after backflushing. It is possible to perform full range hydrogen analysis by just running this channel.
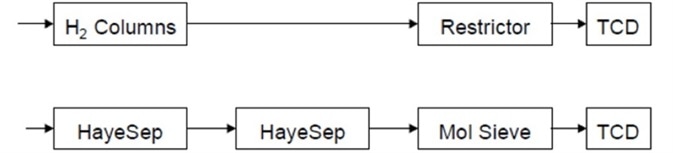
Figure 4. Flow path after the hydrogen channel is switched to backflush. Image Credit: PerkinElmer
The next valve action happens in the light gases channel. The first column is backflushed to vent after oxygen, methane, hydrogen, nitrogen, CO and CO2 have fully eluted from the first HayeSep® column. This flow path is shown in Figure 5.
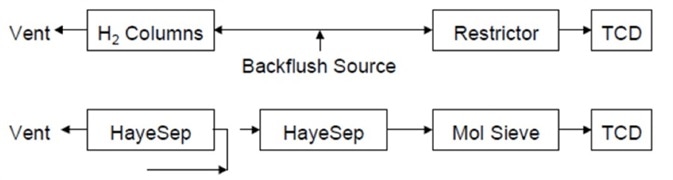
Figure 5. Flow path after backflush of first HayeSep column. Image Credit: PerkinElmer
The action sends all other components to vent and the analyzer stays in this position until hydrogen elutes from the molecular sieve column. This column is bypassed once hydrogen elutes from the molecular sieve column, collecting oxygen, methane nitrogen, and CO in the molecular sieve.
Figure 6 shows the flow path following the capture of the molecular sieve column. Until CO2 has eluted completely, the analyzer stays in this condition. There are no other components in the second HayeSep column once CO2 has fully eluted and the molecular sieve column is returned to the flow path.
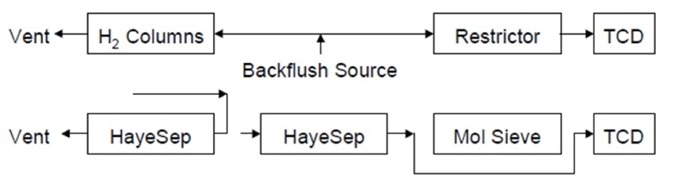
Figure 6. Flow path after capture of the molecular sieve column. Image Credit: PerkinElmer
Figure 7 shows the final flow path and nitrogen, oxygen, methane and CO elute to the detector. This position is also the fill of ready position for the analyzer. Another sample can be run straight away.
An important feature of both the light gases channel and the hydrogen channel is shown in Table 1, the backflush of the sample to vent. This design, coupled with the use of chlorine resistant materials, enables the Chlorine Analyzer to handle corrosive wet samples.
Table 1. Analyzed components and minimum detection limits. Source: PerkinElmer
| Component |
Minimum Detection Limit (Mole%) |
| |
Standard Detector (Included) |
Enhanced Sensitivity Detector (Optional) |
| Helium |
10 ppm |
|
| Hydrogen |
10 ppm |
10 ppb |
| Carbon Dioxide |
100 ppm |
50 ppb |
| Oxygen/Argon |
100 ppm |
50 ppb |
| Nitrogen |
100 ppm |
50 ppb |
| Methane |
100 ppm |
50 ppb |
| Carbon Monoxide |
100 ppm |
50 ppb |
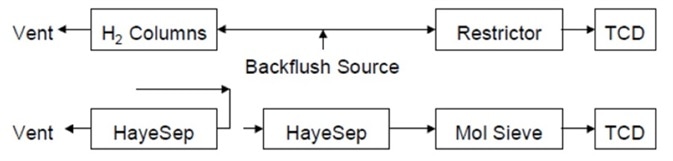
Figure 7. Flow path after the release of the molecular sieve column. Image Credit: PerkinElmer
Water in the sample
Along with handling gaseous samples via the gas sampling loop, as both channels backflush water along with all other components, the Chlorine Analyzer is also designed to measure samples that contain water vapor. Although water condensation in the sampling path or sample loops will adversely affect the sampling results, it will not affect the Chlorine Analyzer.
PerkinElmer chlorine analyzer details
- Exceeds ASTM® E1746
- Chlorine resistance tubes, columns, and valves
- Includes H2 and He analysis in a single run
- Factory pre-configured and tested with guaranteed performance out of the box.
- Flexible, with the optional dual TCD supporting a third analytical channel (i.e., third TCD channel to separate O2 and Ar)
- Sensitive, greater than 10 ppm for H2 and He and 100 ppm for CO2, O2 /Ar, N2, CH4 and CO. With the optional Enhanced Sensitivity Detector, sensitivity levels of 10 ppb for H2, and 50 ppb for CO2, O2 /Ar, N2, CH4 and CO can be achieved
Conclusion
The PerkinElmer Chlorine Analyzer supplies a low cost of ownership. It is an accurate and reliable turnkey solution for measuring chlorine purity, allowing the user to gather vital information about chlorine impurities quickly exceeding ASTM E1746 “Standard Test Method for Sampling and Analysis of Liquid Chlorine for Gaseous Impurities.”
References
- https://www.fda.gov/.
- https://www.epa.gov/.
About PerkinElmer
As a global technology leader, PerkinElmer is taking action to harness the power of insights and transform them into knowledge to deliver innovative, differentiated solutions for our customers.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.