Count to three: “one-one-thousand, two-one-thousand, three-one-thousand”. Three seconds. This is how long it takes for another person to develop dementia somewhere in the world (Alzheimer’s Disease International (ADI), 2019).
Introduction

Image credit: istockphoto.com
Alzheimer’s disease (AD) is the third largest cause of death and disability for older people globally, behind cardiovascular and cerebrovascular diseases and cancers (Xiaoguang Du, Xinyi Wang and Meiyu Geng, 2018). There is currently no treatment to cure Alzheimer's disease or reverse the disease process in the brain although some therapeutics may partially alleviate some of the symptoms. Ultimately severe loss of brain function causes dehydration, malnutrition or infection and this will result in death. Estimates of the current number of people who suffer from the disease range from 35 million to 50 million (WHO, 2020; ADI, 2019), with 10 million new diagnoses expected every year (WHO, 2020). Of these, 58% live in low- and middle-income countries and by 2050 this will be 71% of the total. Eastern Asia and southern Asia will see dementia growth rates more than double in the coming 20 years, Latin America will see increases of 134% to 146% and North Africa and the Middle East can expect a 125% rise (ADI, 2019). The number of people living with dementia globally more than doubled from 1990 to 2016, mainly due to increases in population ageing and growth (GBD 2016 Dementia Collaborators, 2019).
15% of the US population who are over 60 years of age (5.3 million) were estimated to have prodromal AD in 2019, whilst a further 40% of this age group have preclinical AD (Cummings, Feldman and Scheltens, 2019). In the UK, 1 in 14 of those over 65 years of age (850 000) had a diagnosis of dementia in 2019. This has been forecast to almost double to circa 1.2 million people with a specific AD diagnosis by 2040 (Alzheimer’s Society, 2019). 8.8 million in EU-28 were diagnosed with AD across the EU-28 in 2019 (Alzheimer Europe, 2019).
These numbers may well underestimate the true prevalence of the disease as stigma associated with the disease has been associated with concealment of a diagnosis (ADI, 2019). Accurate estimation of the cost associated with managing AD is similarly difficult to do as many of the indirect costs (e.g. those relating to carer expenses and loss of productivity) are often not formally captured. Specifics aside, the AD-related costs are large. Cummings, et al (2019), for example, predicted costs of circa $259 bn in 2017 in the USA alone, rising to an anticipated excess of $1trn by 2050. The global cost is likely to be exponentially higher if the anticipated increase in incidence of AD in eastern Asia, Southern Asia, Latin America and North Africa and the Middle East are taken into account.
Know your adversary
Received wisdom holds that a solid understanding of one’s adversary is necessary before they can be conquered. This is a lesson that everybody globally has had to face up to in some way over recent months in the COVID-19 pandemic. If a similar-sized healthcare and economic crisis centred around AD is to be averted in coming decades, it will be necessary to establish a better understanding of the complex and multifactorial pathways and systems-based interactions that combined to form the heterogenous state that is AD. Alzheimer’s disease is highly complex and poorly understood. Symptoms are defined as cognitive and functional impairment, which become more acute with disease progression.
A cursory glance at the outcomes of clinical trials and studies into AD since 1963 confirms that AD has continued to frustrate researchers’ best efforts to understand the disease and to develop an armoury of diagnostic and treatment tools despite extensive research efforts and targeted funding over the past decades.
The majority of these research efforts have honed in on molecular or biochemical aspects of amyloid-beta plaques, neurofibrillary tangles and cerebral atrophy however many individuals have such features, but never develop the disease. This research has included attempts to understand causal mechanisms and/ or formation processes of these plaques, tangles and/ or atrophy as well as development of pharmacological approaches that prevent formation of or removal of these plaques.
This line of inquiry has however not yet been successful. 99% of Phase 2-3 clinical trials into new therapeutics have failed and a failure rate of 100% has been reported for AD disease modifying therapies (Cummings, et al 2019). In contrast, little research attention has been paid to potential alternative mechanisms of action that might explain the disease in the context of the complexity of the multi-systems neural network.
The Antimicrobial Infection Hypothesis suggests one such alternative explantion. This hypothesis recognises the mutual impact of microbial infection and neuroinflammation on one another and postulates that pathogen interference together with an innate immune system response can be a significant contributor to the development of AD.
This white paper will highlight key hypotheses that have guided AD research and product development to date. The antimicrobial infection hypothesis will then be presented as a feasible explanation for some of AD heterogeneity that may provide a basis for a potentially more personalised intervention approach.
The Alzheimer’s Disease research journey to date
Image credit: istockphoto.com
Although the first clinical case study of AD was presented in 1906 and the term “Alzheimer’s disease” was coined in 1910, theories as to the cause and natural course of this disease only started to gain traction from 1963. Since then, theories revolving around the development and presence of extracellular amyloid-beta plaques and agglomerations of tau protein in brain tissue have taken the lion’s share of the global research attention in this field. Approved interventions for sporadic AD are limited to management of symptoms and only a handful of medications have received regulatory and marketing approval globally (ResearchAndMarkets, 2020). These medications include cholinesterase inhibitors (Donepezil, Rivastigmine and Galantamine), N- methyl-D-aspartate (NMDA) receptor antagonists (Memantine), an A A4 protein inhibitor (sodium oligomannate), or a combination of these (Memantine/Donepezil) (Xiaoguang Du, et al, 2018). A further 11 late stage pipeline products were proposed for launch in the coming months over the period 2020/ 2021 (ResearchAndMarkets, 2020). Disease modifying therapies are currently under development to target amyloid-beta levels in people with familial AD. These include fully human and humanised monoclonal antibodies (Aducanumab, Gantenerumab, Solanezumab). Their efficacy remains to be demonstrated.
The gaps
Much remains to be clarified and explained in our understanding of the underlying aetiology of AD. Whilst the initial hypothesising around the pathogenesis of AD was predicated on the back of strong genetic, biochemical and histopathological findings in the form of the amyloid-beta cascade- and tau hyperphosphorylation hypotheses (Fan, et al 2020), these outputs have not translated well into therapeutic interventions for the disease. Continued lack of clarity around causal factors, processes and end effects hampers design and development of drugs and other interventions that would allow patients to be more independent in everyday life, slow down the progression of the disease and, ideally, work towards a cure.
The vast majority of explanations to date for the aetiology and pathogenesis of AD have been based on the amyloid cascade- and tau hyperphosphorylation hypotheses (Fan, et al, 2020). Explanations for the disease have to date therefore been couched in biochemical concepts around gene expression, neurotransmitter functioning or pathways that contribute to the formation of amyloid plaques, neurofibrillary tangles and cerebral atrophy. Approximately 40% of clinical trials, for example, have focused on the amyloid and neurotransmitter hypotheses (Xiaoguang Du, et al, 2018).
Details of both established and emergent hypotheses that have been proposed to explain AD pathogenesis are summarised in Table 1 and Table 2, respectively. An overview of the theoretical stance taken across AD clinical trials up to 2019 is shown in Figure 1.
Table 1 Summary of established hypotheses for Alzheimer’s Disease pathogenesis
|
Hypothesis
(originator)
|
Essence of the hypothesis
|
Contribution
|
Limitations
|
|
Cholinergic*, **
(Peter Davies and A.J.F. Maloney, 1976)
|
Inhibition of acetylcholine degradation to improve cognitive functioning.
|
First demonstration of centrality of cholinergic system failure as central in AD.
|
Temporary symptomatic treatment only;
Unable to slow disease progression.
|
|
Amyloid*, **
(John Hardy and David Allsopp, 1991)
|
A pathogenic mutation in the amyloid-beta precursor protein (APP) gene on chromosome 21 has been linked with deposition of amyloid-beta plaques and/ or aggregations of tau protein in the brains of some people with AD. A causal link is being questioned between amyloid aggregations and AD symptoms.
Amyloid-beta is viewed as an inherently abnormal by-product that accumulates in the brain with age in this hypothesis. High concentrations of amyloid-beta are neurotoxic and cause dendritic and axonal atrophy in mature neurons and ultimately cell death.
Current thinking suggests amyloid-beta should be treated purely as a pathological feature rather than from a mechanistic perspective.
|
This hypothesis shifted the explanation of AD from being descriptive to mechanistic.
|
Treatment frequently worsened cognition and induced side effects.
|
|
Immunotherapy**
|
Use of monoclonal antibodies to bind and/ or trigger clearing of soluble amyloid-beta.
|
To be determined once this course of intervention has been approved and implemented.
|
No approved mAbs yet for AD.
|
|
Neurotransmitter modulation*, **
|
Modulation of neurotransmitters to improve cognitive function (e.g. acetylcholine, N-methyl-D-aspartate) to sustain the action of the neurotransmitter within the synaptic cleft.
|
Acknowledges the critical role of acetylcholine and cholinergic neurons in the physiological processes of attention, learning, memory, stress response, wakefulness and sleep.
|
Symptomatic treatment means that effects are short-lived;
Side effects are often experienced, including impaired learning and memory as a result of synaptic dysfunction and induced hyperphosphorylation of tau protein as a result of oxidative damage to synaptic membranes.
|
|
Tau propagation*, **
(Clavaguera, et al, 2009)
|
Tau-containing neurofibrillary tangles are a pathological feature of AD. Hyperphosphorylated tau protein aggregations depolymerise microtubules and impact signal transmission within and between neurons. This potentially results in iron accumulation in neurons.
|
Yet to be confirmed.
|
Few agents in drugs targeting the tau protein have established proof-of-principle;
Effectiveness of drugs in this hypothesis class not yet tested;
Likely to face similar challenges to amyloid-beta drug trials.
|
|
Mitochondrial cascade and related hypotheses*, **
(Swerdlow and Khan, 2004)
|
- Mitochondrial dysfunction may impact the expression and processing of APP and the accumulation of amyloid-beta in Sporadic AD. Mitochondrial damage results in increased reactive oxygen species (ROS) production;
-Epigenetic dysregulation;
-Impaired mitophagy in AD
|
May be a link between cholinergic hypothesis, amyloid hypothesis and tau propagation hypothesis.
|
Reduced glucose metabolism is a result rather than a cause of amyloid-beta accumulation;
No treatment strategy or approach is suggested by this hypothesis.
|
|
Calcium homeostasis*, **
(Mattson, et al, 1992)
|
Calcium levels in neurons can be elevated by amyloid-beta, making the neurons more vulnerable to environmental stimuli.
|
Calcium homeostasis has been associated with learning and memory.
|
Rating of the effectiveness of drugs based on this hypothesis (e.g. Memantine) as low.
|
|
Neurovascular*, **
|
Disruption to the delivery of substrates and removal of waste results in brain dysfunction more generally and is associated with impaired microcirculation, hypoperfusion, hypoxia and neurovascular inflammation. This may play a role in the development of AD.
|
Impacts of hyperlipidaemia and hyperglycaemia recognised on the progression of AD.
|
May only partially explain how AD is caused.
|
|
Inflammatory*, **
|
Inflammatory factors released by microglial cells result in inflammation. Higher activity in microglial cells of AD patients has been observed.
|
Impacts of inflammatory responses of microglia and astrocytes recognised on development of AD.
|
Further clarification of details of this hypothesis may be warranted before other anti-inflammatory AD drug development is embarked upon.
|
|
Metal ion*
|
Dyshomeostasis of biometals in the brain interferes with several brain functions. Increased or decreased levels of zinc, copper and iron have been highlighted in AD brains and the effect of aluminium needs to be clarified. Biometals may influence AD pathogenesis either directly or indirectly.
|
Could have an effect on oxidative stress (Wang, Yin and Liu, 2020).
|
Incomplete explanations are provided by this hypothesis (e.g. effect of aluminium on amyloid-beta burden needs to be clarified).
|
|
Lymphatic system*
|
Age-related impairments in vascular and peri-vascular removal of waste products through the CSF result in accumulation of amyloid-beta in the brain meninges.
|
Cognisance is given to the importance of clearance of amyloid-beta in addition to reducing deposition.
|
Further validation of potential mechanisms and pathways is necessary.
|
|
Endocrine pathway and vagus nerve as part of the gut-microbiota-brain axis**
|
Close functional links exist between the gut as the body’s largest endocrine organ, it’s microbiota and the vagus nerve as a peripheral end point of the central nervous system.
Hormone secretion impacting on brain functioning (e.g. corticosterone and adrenal hormones) arises from the gut and facilitates intestinal-brain information exchange between neurotransmitters including dopamine and acetylcholine that are produced in the gut and transmitted through the vagus nerve to the central nervous system.
|
This hypothesis holds potential for new insights to be generated that can be translated into therapeutic avenues for AD (e.g. stimulation of the vagus nerve to attenuate pathophysiological changes and modulate homeostasis.
|
Further clarification is needed of the feasibility of a causal relationship between gut-microbiota-brain axis- related inflammation and amyloidosis.
|
|
Harnessing of bacteria-derived metabolites**
|
Utilisation of naturally occurring microbiome metabolites (e.g. short chain fatty acids) to modulate peripheral and central pathological processes (e.g. reducing inflammation, modulate signalling pathways, production of proinflammatory cytokines that are associated with deposition of amyloid-beta and pathogenesis of AD).
|
This hypothesis could feasibly provide an alternative explanation for the inflammatory component that is found in AD as well as form the basis for development of a sustainable intervention for AD.
|
High quality clinical trials have not yet been carried out to validate the theoretical basis for using microbiota-directed therapies to ameliorate symptoms of AD and to clarify causality in the relationship between GMB-related inflammation and amyloidosis.
|
* Liu, P., Xie, Y., Meng, X. and Kang, J.-S. (2019) ** Xiaoguang Du, et al 2018
Table 2 Emerging alternate explanatory theories for AD***
|
Gamma oscillations
|
Activation of local excitatory circuits and fast-spiking inhibitory neurons by rhythmic fluctuation of brain waves appear to be associated with higher order cognitive functions including memory formation and selective attention.
|
Potential to manipulate neural network oscillation disturbances presents an alternative approach from which to develop an intervention for AD.
|
Investigations have not yet been translated from animal to human studies;
It is not yet clear how gamma oscillations relate to initial deposition and/ or clearance of amyloid-beta in neurons. This hypothesis may relate to development of AD interventions rather than describe the initial pathogenesis of the disease.
|
|
Prion transmission
|
Amyloid-beta and tau protein appear to spread between brain regions with a similar pathogenic conformation to prion protein by cross-synaptic transmission.
|
Recognition of the acknowledged effects of prion protein on neurodegeneration (e.g. Creutzfeldt-Jakob Disease).
|
Further studies are required before the concept in this hypothesis can be applied to development of a therapeutic for AD.
|
|
Interaction between amyloid-beta and hippocampal ghrelin/GHSR1α signalling
|
GHSR1α (Ghrelin receptor signalling contributes to learning and memory processes in the hippocampus. Early findings suggest it may contribute to neurogenesis by attenuating hippocampal pathology, has links with hippocampal metabolic processes and calcium signalling, may indirectly drive hippocampal damage and affect hypothalamic function.
|
Hippocampal lesions appear early in AD. GHSR1α may therefore be a novel target for AD intervention.
|
Further studies are required to characterise these GHSR1α signalling deficits more fully in the brains of people with AD;
Does not prevent hippocampal lesions or functional cognitive losses (Tian, Wang, Wang, Guo and Du, 2019).
|
|
Cerebral vasoconstriction
|
Reduced cerebral blood flow and angiogenesis damage are proposed as the initial two changes in brain function in AD (Zhu et al, 2012). Capillary contraction along with significantly reduced grey blood flow promotes amyloid-beta production that in turn generates reactive oxygen species (ROS) and evokes pericyte contraction.
|
This is a proposed novel mechanism of action to explain vasoconstriction processes in AD. As such it may provide a new avenue for intervention in AD.
|
Early stage research needs expansion and validation before it can be taken into the clinic.
|
|
Infection*, ***
|
Amyloid-beta has been shown to be an antimicrobial peptide with the function of protecting the brain from microbial agents (e.g. HHV-6A, Porphyromonas gingivalis, Candida albicans) in healthy brains. APP mutation may result in a loss of this biological function and lead in turn to further infections and/ or a decreased innate immune function.
|
This hypothesis introduces the possibility to explain AD pathogenesis beyond the biochemical and genetic perspectives that have been used to date.
|
Experimentation in this hypothesis is as yet early stage and requires translation into in vivo and human trials.
|
*** Fan, et al (2020)
Figure 1 Distribution of proposed theories of AD aetiology in clinical trials prior to 2019*
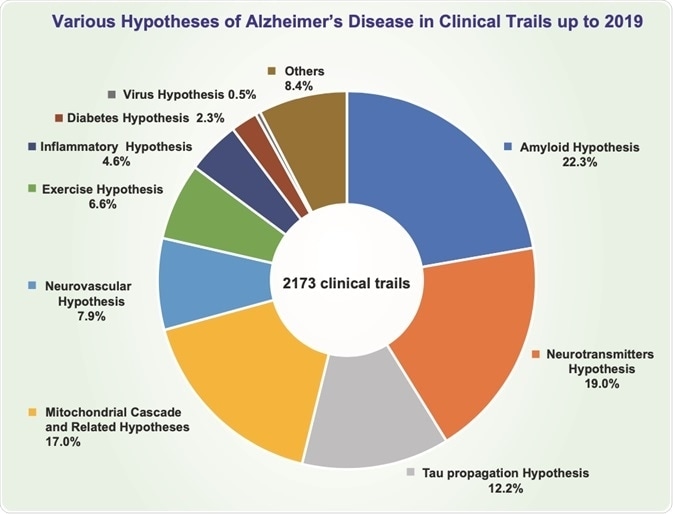
Figure credit: Liu, P., Xie, Y., Meng, X. and Kang, J.-S. (2019) “History and progress of hypotheses and clinical trials for Alzheimer’s disease”, Signal Transduction and Targeted Therapy, 4(29), https://doi.org/10.1038/s41392-019-0063-8.
Antimicrobial Infection Hypothesis
The proposition
This relatively recent hypothesis takes the well-established AD feature of neuroinflammation as its starting point and aims to evaluate the proposition that AD-related changes are triggered by a microbial agent. Early findings in studies have suggested several immune-related genetic risk factors that could contribute to the inflammatory process and noted increased production of cytokines in AD (Shi, Sabbagh and Vellas, 2020). Microbial infections (e.g. HHV-6A human herpes virus infections, Porphyromonas gingivalis infections) have been linked to AD. Similarly, chronic inflammatory diseases (e.g. rheumatoid arthritis) have been associated with a combination of periodontal disease and higher incidences of AD (Fan et al, 2020).
Notwithstanding the possibility that neuroinflammation may be viewed as part of the aging process and may therefore occur independently or in the absence of an infectious agent (Moir, et al, 2018), the antimicrobial infection hypothesis deserves further research attention.
It calls into question the traditional one-size-fits-all approach which has been used to date to understand AD pathogenesis and treat the functional symptoms of the disease. Instead, this hypothesis offers potential for a more personalised approach to managing the disease, potentially guiding a range or combinations of different therapies such as anti-amyloid-beta interventions, anti-inflammatories and/ or anti-infectives as appropriate.
Research gaps to be addressed
Gaps that need to be filled in this existing research base include whether bacterial pathogens exert an initiation and causative effect on development of AD, description of the mechanisms by which local and remote microbial pathogens could potentially impact neurodegenerative processes in AD (e.g. whether innate immune-mediated inflammation plays a role in the propagation of neurodegeneration in AD) and identification of potential candidate therapeutics targeting the regulation and control of pathogen-associated networks and molecules in AD.
A novel programme of research
A UK-based international multidisciplinary consortium has taken up the challenge to address some of these gaps with a submission to the NIH’s "Research on Current Topics in Alzheimer's Disease and Its Related Dementias” research programme. The Biophys Ltd-led microbiome-based proposal was under NIH review at the time of this paper. If successful, this programme of work could open new avenues of exploration for AD with potential to explain discrepant single system findings from previous research. It holds potential to generate invaluable insights into causal relationships in AD, mechanisms of action and translation of these into foundations for interventions.
References
- Alzheimer Europe (2019) Dementia in Europe Yearbook 2019, Luxembourg: Alzheimer Europe.
- Alzheimer’s Disease International. 2019. World Alzheimer Report 2019: Attitudes to dementia. London: Alzheimer’s Disease International.
- Alzheimer’s Society Annual Report (2019) https://www.alzheimers.org.uk/about-us/strategy-and-annual-reports/annual-reports.
- Cummings, J., Feldman, H.H. and Scheltens, P. (2019) The “rights” of precision drug development for Alzheimer’s disease”, BMC, 11: 76, https://alzres.biomedcentral.com/articles/10.1186/s13195-019-0529-5.
- Fan, L., Mao, C., Hu, X., Zhang, S., Yang, Z., Hu, Z., Sun, H., Fan, Y., Dong, Y., Yang, J., Shi, C. and Xu, Y. (2020) “New insights into the pathogenesis of Alzheimer’s Disease”, Frontiers in Neurology, 10 (Article 1312), https://www.frontiersin.org/articles/10.3389/fneur.2019.01312/full.
- GBD 2016 Dementia Collaborators (2019) “Global, regional, and national burden of Alzheimer's disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016” The Lancet Neurology, 18(1):88-106.
- Jing Tian, Tienju Wang, Qi Wang, Lan Guo and Heng Du (2019) “MK0677, a Ghrelin mimetic, improves neurogenesis but fails to prevent hippocampal lesions in a mouse model of Alzheimer’s disease pathology”, Journal of Alzheimer’s disease, 72(2); 467-478, doi: 10.3233/JAD-190779, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7153492/.
- Liu, P., Xie, Y., Meng, X. and Kang, J.-S. (2019) “History and progress of hypotheses and clinical trials for Alzheimer’s disease”, Signal Transduction and Targeted Therapy, 4(29), https://doi.org/10.1038/s41392-019-0063-8.
- Moir, R.D., Lathe, R. and Tanzi, R.E. (2018) “The antimicrobial protection hypothesis of Alzheimer’s disease”, Alzheimer’s & Dementia, 14(12): 1602-1614.
- Shi, J., Sabbagh, M.N. and Vellas, B. (2020) “Alzheimer’s disease beyond amyloid: strategies for future therapeutic interventions”, BMJ, 371, m3684 doi:10.1136/bmj.m3684.
- Wang, L., Yin, Y.L., Liu, X.Z., Shen, P., Zheng, Y.G., Lan, X.R., Lu, C.B. and Wang, J.Z. (2020) “Current understanding of metal ions in the pathogenesis of Alzheimer’s disease”, Translational Neurodegeneration, 9(10), https://translationalneurodegeneration.biomedcentral.com/articles/10.1186/s40035-020-00189-z.
- Wang, W., Zhao, F., Ma, X., Perry, G. and Zhu, X. (2020) “Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: recent advances”, Molecular Neurodegeneration 15(30), https://doi.org/10.1186/s13024-020-00376-6.
- WHO (2020) https://www.who.int/news-room/fact-sheets/detail/dementia#:~:text=Worldwide%2C%20around%2050%20million%20people,60%E2%80%9370%25%20of%20cases.
- Xiaoguang Du, Xinyi Wang and Meiyu Geng (2018) “Alzheimer’s disease hypothesis and related therapies”, Translational Neurodegeneration, 7:2, DOI 10.1186/s40035-018-0107-y.
- Zhu, X.H., Qiao, H., Du, F., Xiong, Q., Liu, X., Zhang, X., Ugurbil, K. and Chen, W.(2012) “Quantitative imaging of energy expenditure in human brain”, Neuroimage, 60:2107–17. doi: 10.1016/j.neuroimage.2012.02.013
About Biophys Ltd.
Biophys Ltd is a consultancy that works with medical device and biotechnology businesses to create solutions to technical, business management and leadership challenges. These solutions can include overseeing commercial progress, providing interim management to oversee periods of change, project management of research, infrastructural or business critical aspects, providing input into IP strategy and development, technical strategy development, contributing to and troubleshooting in product development, advising on biosafety and biosecurity and production of corporate and scientific communications to establish their brand.
The company was born in South Africa in 1996 as a contract research organisation to raise funds for and the profile of early career researchers. Initial expertise in food processing and aquaculture broadened into the life sciences sector with extensive work with enzyme-based diagnostics, natural product purification, small molecule drug development for therapeutics and involvement with product development, quality management, risk assessment and regulatory conformity for lateral flow diagnostics, chemical diagnostics, dry inhalers, transdermal drug delivery devices and injectable medical devices.
We know that market development is just as important as these technical research and development and have therefore assisted medical device and biotechnology companies to evaluate potential markets, position their brand in the UK life sciences arena and, where appropriate, to access available funding.
This information has been sourced, reviewed and adapted from materials provided by Biophys Ltd.
-1-1-1-1.jpg)
For more information on this source, please visit Biophys Ltd.