For many decades, Alzheimer’s researchers have struggled with a “chicken or egg” question regarding the proteins commonly associated with the condition.

Image Credit: Charles River Laboratories
It is accepted that the overproduction or decreased clearance of amyloid beta (a protein associated with neural repair and growth) forms hard plaques in the brains of Alzheimer's disease (AD) patients, and that tau protein, found in brain cells, can become toxic, forming tangled spaghetti-like strings.
The problem of whether an abnormal build-up of amyloid plaques or dysfunctional tau drives neurodegeneration in the brains of patients with AD, or whether these are consequences of an exceptionally complex disease process has not been confirmed by researchers.
Dr. Rudolph Tanzi, Professor of Neurology at Harvard and the Director of the Genetics and Aging Research Unit at Massachusetts General Hospital (MGH), believes that amyloid is the main driver of AD. In the 1980s, his team (alongside others) identified the gene making beta-amyloid on chromosome 21, where rare mutations can cause early-onset familial AD. The gene codes for amyloid beta-protein precursor (APP) can lead to increased levels of amyloid beta peptides in the brain. The longer and more adhesive forms of this peptide can accumulate causing amyloid plaques, a characteristic of AD.
Tanzi believes in the amyloid hypothesis, but realized in time that therapeutic targeting of these toxic proteins in the brains of AD patients would be insuffient to prevent this disease/ For disease prevention, intervention must be executed much earlier, ideally before the appearance of symptoms.
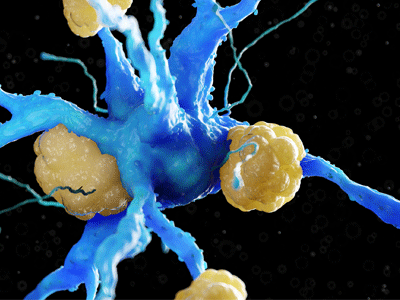
Amyloid plaques on a neuron. Image Credit: Charles River Laboratories
Neuroinflammation in AD
While tau tangles and amyloid plaques inflict damage in the early stages of disease, most damage is arguably caused by specialized immune cells (malfunctioning microglia) in the central nervous system which trigger near-continuous neuroinflammation.
Generally, microglia displace neurological debris (including amyloid residue). However, in AD they go into overdrive, destroying healthy neurons and accelerating the course of the disease. Many scientists believe the chronic immune response is the third core pathological feature of AD.
In 2008, Tanzi’s lab linked CD33 to neuroinflammation: CD33 is a surface cell receptor found on microglia and other immune cells that is more abundant in AD patients. Increased levels of CD33 are associated with disease severity. Five years later, Tanzi found that highly expressed CD33 can damage neurons by signaling microglia to initiate neuroinflammation.
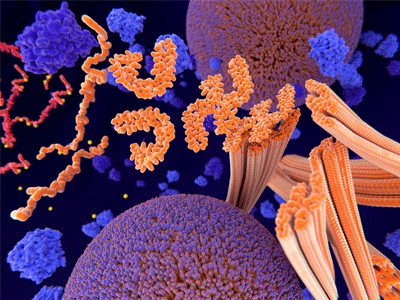
Tau protein in red and yellow. Image Credit: Charles River Laboratories
TREM2, another gene expressed by microglia, also plays a complex role in neuroinflammation. Usually it encodes for a protein regulating microglia response to infection and injury, including clearing excessive tau and amyloid from the brain. However, in 2012 a University of London scientist discovered that variations actually decrease the function of the protein, potentially increasing the risk of AD threefold.
Tanzi’s lab published animal data demonstrating how the crosstalk of CD33 and TREM2 can play a role in neuroinflammation, where CD33 turns it on and TREM2 shuts it down. Tanzi stated that if CD33 is silenced but TREM2 is maintained, the toxic inflammation that accelerates AD can potentially be reduced. In Phase III trials for AD about 20% of the disease-modifying drugs have immunomodulating actions targeting microglia, the vast majority of which are aimed at TREM2.
Multiple targets of neuroinflammation
Dan Rocca, PhD, a Research Leader at Charles River Laboratories, says that microglia undoubtedly have a complex role within Alzheimer’s disease, and that there can be difficulty determining their behavior over the progression of the disease. “Depending on what stage you're at, microglia can be either exacerbating the disease, or protecting you against it and alleviating some of the symptoms,” says Rocca. “They can potentially throw out lots of cytokines, which cause inflammation and death of neurons, but they can also engulf amyloid beta and so reduce those plaques.”
While TREM2 and CD33 are clear targets for drug developers, other avenues of research exist. Rocca says some companies are targeting the inflammasome, danger-sensing proteins that are triggered when a cell is infected or damaged. There is evidence that both beta amyloid and tau in the brain can prompt persistent activation of the NLRP3 inflammasome, causing chronic neuroinflammation.
“There are companies that have developed small molecules that have been modified chemically to penetrate the brain. They work by targeting the inflammasome to potentially stop the adverse effects of microglia,” says Rocca.
Pharmaceutical companies are currently searching for preclinical models to demonstrate microglial activation, says Dr. Susanne Bäck, PhD, Senior Manager of CNS Pharmacology at Charles River’s Kuopio, Finland site. Several mouse models exist that can be employed to measure neuroinflammation in AD, says Dr. Bäck. “But it’s difficult because in most models, neuroinflammation is mainly a secondary response to amyloidβ overproduction and plaque formation and does not capture all inflammatory changes seen in human Alzheimer's."
The Future of AD Therapeutics
Tanzi says a possible avenue for controlling Alzheimer’s treatment is a mixture of drugs, including a therapeutic antibody to clear the plaques and a small-molecule drug, such as the gamma secretase modulator, to minimize amyloid levels. When combined with other drugs, these could control the spread of tangles and reduce neuroinflammation. “But, for this to work, it would be best to give the antibody before people have symptoms,” says Tanzi.
About Charles River Laboratories
At Charles River, we are passionate about our role in improving the quality of people’s lives. Our mission, our excellent science and our strong sense of purpose guides us in all that we do, and we approach each day with the knowledge that our work helps to improve the health and well-being of many across the globe.
Charles River provides essential products and services to help pharmaceutical and biotechnology companies, government agencies and leading academic institutions around the globe accelerate their research and drug development efforts.
As a fully integrated partner, Charles River can support your research at any point along the drug discovery continuum.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.