A deep dive into the brain’s command center for hunger shows how decoding its circuits could transform the future of weight loss treatments.
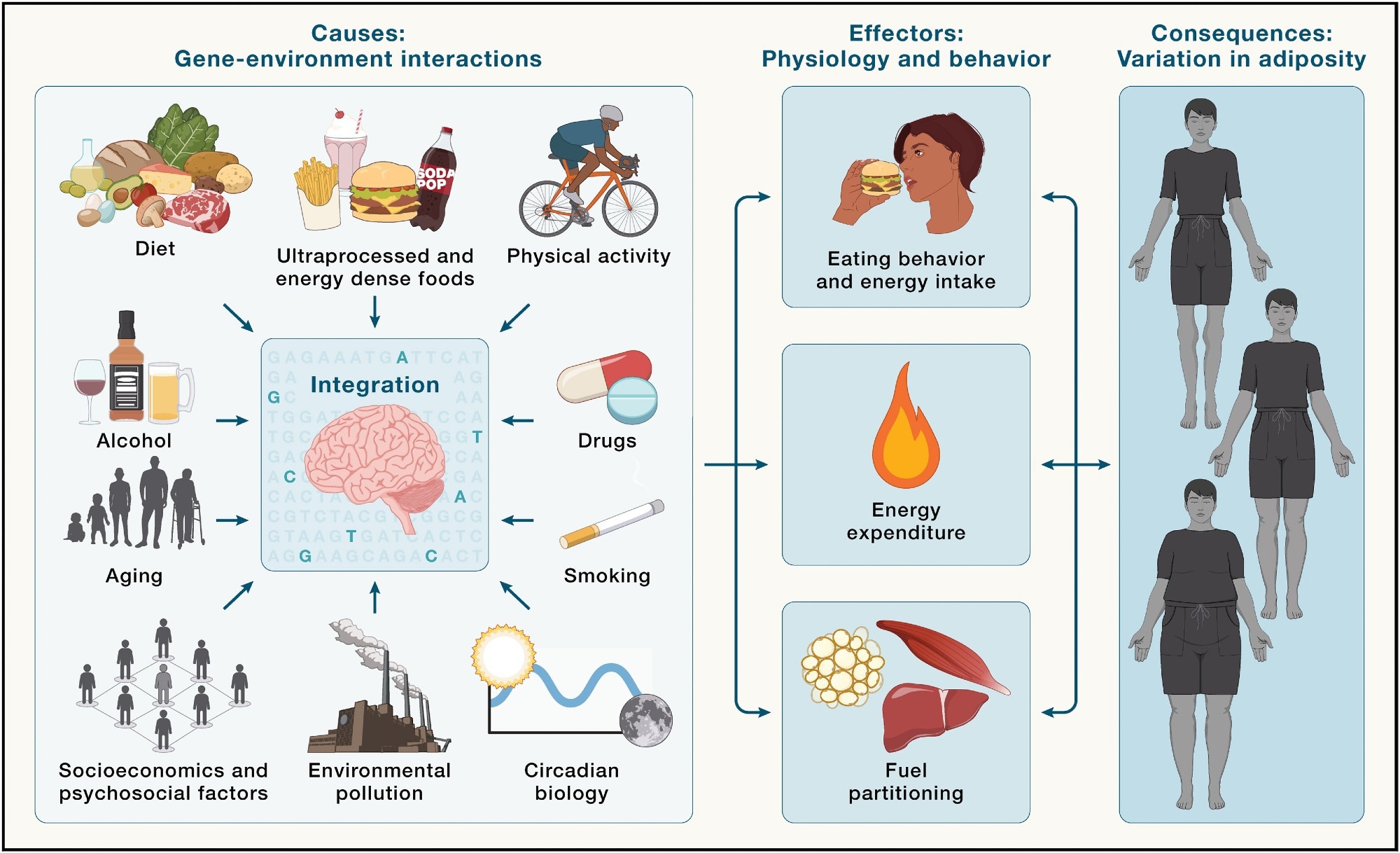
Brain integration of environmental cues in the regulation of energy balance and adiposity - Environmental factors (left) are integrated by the brain in the context of an individual's genetic makeup (illustrated by highlighted SNPs) and epigenetic profile. In response, the brain regulates behavioral and physiological outputs, such as energy intake, energy expenditure, and fuel partitioning (middle), which all influence energy balance, ultimately shaping interindividual variation in adiposity (right).
In a recent review published in the journal Cell, a group of authors synthesized how the central nervous system (CNS) integrates neuroendocrine signals to govern energy homeostasis and translate these mechanisms into safe, effective anti-obesity pharmacotherapy.
Background
How did a survival system become a global health crisis? Since the 1980s, obesity rates have surged, now affecting around one billion people worldwide, with cardiovascular disease driving most obesity-related deaths. Genetics and environment interact: some bodies are primed to gain weight ("drifty gene" hypothesis), others resist, and modern food cues and stressors amplify the gap.
Obesity pathogenesis involves "push" (brain-driven hyperphagia) and "pull" (peripheral fuel sequestration) mechanisms. Meanwhile, brain circuits evolved to defend energy stores, not today’s ultra-processed diets. Understanding how the brain, gut, adipose tissue, and liver converse is key to developing safe, effective anti-obesity therapies. Further research is needed to map neural circuits and activity-dependent neuroplasticity that enable long-term, non-aversive weight loss.
Neuroendocrine Map of Energy Homeostasis
The brain arbitrates energy balance by blending slow adiposity signals with fast, meal-linked cues. Adipose tissue relays status through leptin, while the gastrointestinal (GI) tract releases hormones like glucagon-like peptide-1 (GLP-1), glucose-dependent insulinotropic polypeptide (GIP), cholecystokinin (CCK), peptide YY (PYY), secretin, and ghrelin (the only orexigenic hormone in this list) from the stomach to stimulate appetite via agouti-related peptide (AgRP) neurons. These endocrine signals complement vagal and spinal afferents that sense gut distension and nutrients, giving the CNS rapid feedback.
Together, endocrine and neural inputs coordinate digestion, satiety, and metabolic homeostasis. The liver adds its voice, fibroblast growth factor 21 (FGF21), insulin-like growth factor 1 (IGF-1), and liver-expressed antimicrobial peptide 2 (LEAP2), while small metabolites and bile acids round out the message.
Hypothalamic Circuits: Setting the Energy Budget
The arcuate nucleus (ARC), adjacent to the median eminence (a circumventricular organ (CVO)), has privileged access to circulating hormones and metabolites. ARC neurons express receptors for leptin, ghrelin, and insulin and receive inputs from the paraventricular hypothalamus (PVH), ventromedial hypothalamus (VMH), and dorsomedial hypothalamus (DMH), and extrahypothalamic hubs such as the bed nucleus of the stria terminalis (BNST) and nucleus of the solitary tract (NTS).
Hunger-promoting AgRP neurons release γ-aminobutyric acid (GABA), neuropeptide Y (NPY), and AgRP to inhibit satiety circuits, while pro-opiomelanocortin (POMC) neurons release α-melanocyte-stimulating hormone (α-MSH) to activate melanocortin 4 receptor (MC4R) neurons and curb intake. Synaptic plasticity in these circuits (e.g., leptin-dependent reorganization of inputs) adapts to energy states. These ARC outputs project broadly, allowing hypothalamic networks to set appetite and expenditure.
Hindbrain & Vagal Control: Satiety Without Sickness
The dorsal vagal complex (DVC) integrates visceral signals to terminate meals. In the NTS, calcitonin receptor (CALCR) neurons, including a prolactin-releasing peptide (PRLH) subset, suppress feeding without aversion and can restrain AgRP-driven hunger via polysynaptic pathways.
By contrast, area postrema (AP) circuits can pair appetite suppression with malaise: growth differentiation factor 15 (GDF15) acts via glial cell line-derived neurotrophic factor (GDNF) family receptor alpha-like (GFRAL) neurons that activate parabrachial calcitonin gene-related peptide (CGRP) cells.
Region-specific glucagon-like peptide-1 receptor (GLP-1R) signaling matters: GLP-1R action in the AP drives aversion, whereas GLP-1R action in the NTS promotes satiety, suggesting why some drugs feel smoother than others.
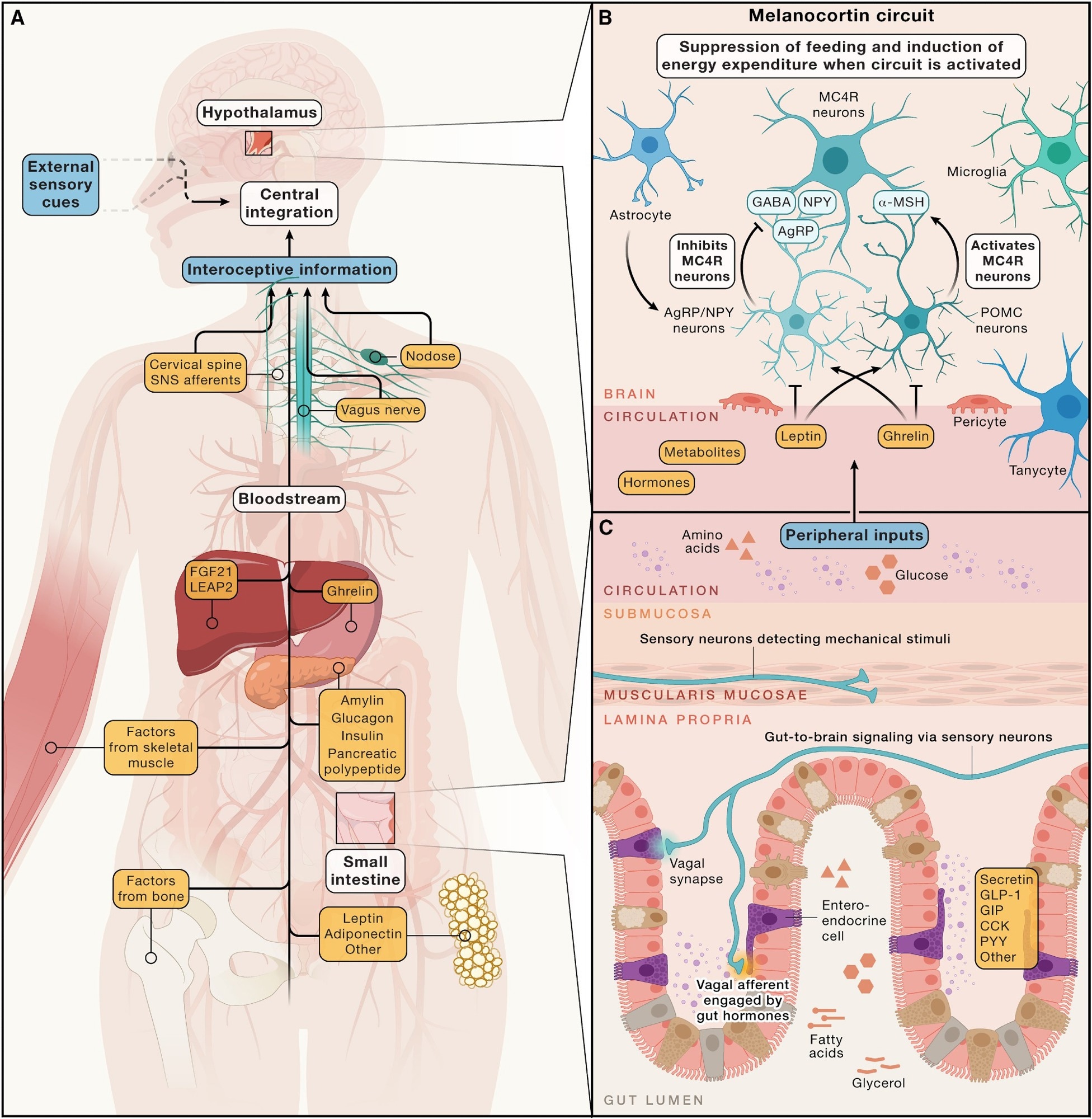
Hormonal, metabolic, and neural inputs to brain circuits regulating energy homeostasis - (A) Simplified overview of peripheral signals arising from multiple organ systems, along with sensory cues from the external environment. These signals are integrated by the central nervous system to regulate energy intake and expenditure, maintaining stable adiposity over time. This regulation involves both long-term energy storage signals, such as leptin, and short-term signals related to immediate intake of energy, like gastrointestinal hormones and nutrients. (B) The arcuate nucleus of the hypothalamus harbors the melanocortin circuit, which is highly responsive to deviations in circulating hormones (e.g., those from the gastrointestinal tract) and the adipose tissue and metabolites. Central to this circuit are hunger-promoting AgRP neurons and satiety-promoting POMC neurons. These neuronal populations modulate energy balance via inhibitory and excitatory inputs to downstream MC4R-expressing neurons, respectively. (C) Many peripheral inputs influencing brain circuits that regulate energy balance originate in the gut. Enteroendocrine cells release hormones, like secretin, GLP-1, GIP, CCK, and others, into the circulation in response to various stimuli, such as the presence of luminal nutrients. Additionally, vagal afferents relay mechanical and chemical information—such as gut distension and nutrient content—from the gastrointestinal tract to the brain.
Motivation & Reward: Why Palatable Foods Win
Mesocorticolimbic pathways, ventral tegmental area (VTA) dopamine projections to the nucleus accumbens (NAc) and prefrontal cortex, assign incentive salience to food cues. The lateral hypothalamus (LH) interfaces with these reward circuits through melanin-concentrating hormone (MCH) and orexin neurons, which project to the VTA and NAc and bias seeking of palatable foods. Because homeostatic and hedonic systems interlock, effective therapies must dampen the drive to eat without flattening everyday motivation. Gut-to-brain signaling via the vagus can activate dopamine neurons after sugar sensing, helping explain why ultra-processed foods feel compelling even without strong taste cues.
From Circuits to Medicines: What Works Now
Earlier drugs worked mainly through monoamines (dopamine, norepinephrine, serotonin), as in phentermine–topiramate or bupropion–naltrexone, yielding about 8%-10% loss with cardiovascular, GI, and psychiatric trade-offs. Peptide engineering changed the game: reversible albumin binding extended incretin half-life, enabling GLP-1 therapies; liraglutide produced a 5.4% placebo-corrected loss at 56 weeks in people with obesity without diabetes.
Co-engaging glucose-dependent insulinotropic polypeptide receptor (GIPR) can blunt GLP-1R–linked aversion while preserving intake suppression, one plausible reason dual incretins achieve greater weight loss. Amylin receptor (AMYR) agonists, which act through CALCR–receptor activity-modifying protein (RAMP) complexes in the AP and ARC, also suppress intake and may do so with fewer aversive signals. Notably, the single-molecule GLP-1R/AMYR co-agonist amycretin drove 24% weight loss in a phase 1/2 trial.
Knowledge Gaps with Real-World Stakes
Key unknowns include which neuron populations sustain non-aversive satiety, how diet and stress reshape synapses in hypothalamic, hindbrain, and reward networks, and how to ‘rewire’ maladaptive circuits safely. Answering these questions would refine patient-specific therapy (choice of incretin backbone, amylin receptor add-ons), reduce discontinuation, and extend cardiometabolic benefits for families and health systems for many patients.
Conclusions
To summarize, the brain is the command center of energy homeostasis. By integrating endocrine signals from adipose tissue, the GI tract, the pancreas, and the liver with rapid neural input, central circuits, from ARC melanocortin pathways to DVC and mesocorticolimbic networks, set appetite, expenditure, and reward.
Peptide pharmacotherapies that target GLP-1R, GIPR, and amylin pathways already deliver meaningful weight loss. Future success will depend on mapping activity-dependent neuroplasticity and designing combinations that maximize satiety, minimize aversion, and protect long-term cardiometabolic health for people everywhere.
Journal reference:
- Johansen, V. B. I., Petersen, J., Lund, J., Mathiesen, C. V., Fenselau, H., & Clemmensen, C. (2025). Brain control of energy homeostasis: Implications for anti-obesity pharmacotherapy. Cell, 188(16), 4178–4212. DOI: 10.1016/j.cell.2025.06.010 https://www.cell.com/cell/fulltext/S0092-8674(25)00677-4