Sponsored Content by ESSLABReviewed by Olivia FrostAug 28 2025
Tired of the limits that come with purchasing conventional reference materials from stock catalogs? Sifting through endless catalogue pages with varying concentrations and matrices wastes valuable time, with no guarantee of finding the standard you need.

Image Credit: ESSLAB
Many chemists avoid the tedious process entirely, instead mixing their own analytical standards in-house. However, this approach adds extra workload and considerable risk.
Luckily, there is a better solution. Custom certified reference materials (CRMs) from Inorganic Ventures are a simpler and faster approach to obtain the appropriate analytical standard reference material for your unique use case. Inorganic Ventures always encourages its clients to share the responsibility for establishing calibration standards.
Allow Inorganic Ventures to handle the burden of creating custom analytical standards depending on your specific requirements. This saves time and removes the risks of blending standards by hand.
Not sure that custom CRMs outperform analytical standards mixed in-house? Here are six reasons why you should trust Inorganic Ventures to create calibration solutions for your workflow rather than blending them yourself.
1. Mixing analytical standards is expensive
Combining single-element analytical standards into a viable solution requires both resources and time. Consider the administrative, equipment, and human costs of stocking and measuring reorder points per solution. These costs mount quickly into a significant ongoing expense.
Inorganic Ventures' analytical standards ensure that you pay only for what you need, making inventory management much easier. Its CRMs are ready for instant use without any additional preparation, and each standard can be diluted directly from the bottle.
2. Documenting analytical standards is a pain
When creating your own calibration standards, you are always responsible for providing verification.
Labs held to strict testing specifications must continuously ensure that every standard is generated to the highest possible standards. This requires a high level of confidence and organization. An audit can turn this into a high-pressure test of record-keeping.

Image Credit: ESSLAB
With a custom CRM, all regulatory documents, including CoA and SDS, are prepared for you and ready for inspection. Its standards are manufactured and certified in accordance with ISO 17025 and ISO 17034 Scopes.
3. Human error is a real risk
To err is human, and even experienced scientists make mistakes. When mixing analytical standards in-house, you will inevitably second-guess the validity of your data. Unfortunately, there is no margin for error in the analytical sector.
Mixing errors, whether in amounts, matrix composition, or other factors, can quickly undermine accuracy.

Image Credit: ESSLAB
Common human errors that can jeopardize DIY standards include:
- Incorrect calculations or dilutions.
- Inadvertently using the incorrect standard can result in incorrect concentrations.
- Improper matrix use may result in interferences.
- Inadequate knowledge of stabilizers can lead to an unstable matrix.
- Recording incorrect weight while preparing gravimetrically.
4. Storing analytical standards can pose further problems
Transpiration can progressively raise the concentration of your analytical standards over time. Unfortunately, there is no way to prevent this phenomenon when combining your own working standards in-house, as it is caused by water vapor penetration into the mixture, either through container walls or through the opening.
Inorganic Ventures' proprietary packaging technology minimizes transpiration, preserving CRM integrity for years. Transpiration Control Technology (TCT) prevents transpiration from the bottle by creating an equilibrium within the heat-sealed container.
Analytical standards packaged in this manner keep their integrity for up to four years after manufacturing.
Products can be stored in the TCT bag for up to three years, and then for another year after removal. The time and expense savings associated with regular inventory refilling are completely removed, thus alleviating your storage worries.
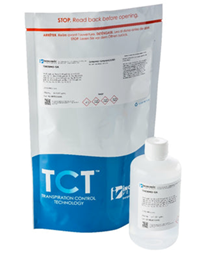
Image Credit: ESSLAB
5. Your lab could be introducing contaminants
Analytical standards must be delivered in ultra-clean bottles, and the CRM is prepared using sterile laboratory equipment and clean beginning materials. It is possible to reproduce these stringent cleaning conditions in your own lab, but this is just another example of the work and cost implications of mixing calibration standards oneself.
The Inorganic Ventures team conducted comprehensive leaching experiments to determine contamination levels in all materials used to produce the CRMs. The team obtain the cleanest components (beginning materials, weigh boats, pipettes, bottles, and so on) and correctly leach them all to achieve the cleanest final analytical standard possible.
Inorganic Ventures also has a separate clean room with a HEPA filtration system and ultra-high purity acids for manufacture. This safeguards against environmental contamination at every stage.
6. There is nowhere to turn when faced with unexpected results
If your results are incorrect, it might be incredibly difficult to identify the cause of the error. Simple mistakes, such as going over volume, will necessitate boiling the concentrate, usually in glass, which may introduce more pollutants. The only solution is to start afresh.
ESSLAB’s technical team provides expert support for sample preparation and troubleshooting during testing.
About ESSLAB
ESSLAB (Essex Scientific Laboratory Supplies Limited) is a leading global supplier of analytical consumables for chemical analysis. Founded in 1982, ESSLAB has built a strong reputation in the fields of chromatography, spectroscopy, and electrochemistry. Known for their expertise in precision liquid handling, ESSLAB offers a wide range of high-quality products that go beyond just laboratory instruments.
As a key distributor for some of the world’s top Reference Material producers, ESSLAB provides a full range of Certified Reference Materials (CRMs) — both organic and inorganic. These materials are essential for industries to ensure product quality, meet regulatory standards, and improve processes. ESSLAB’s commitment to quality has earned it a reputation as a "gold standard" supplier, trusted by customers worldwide.
The company is also known for its team of highly skilled specialists, who offer expert advice and customized solutions to help clients meet their specific needs. Whether it's navigating complex technical challenges or choosing the right products, ESSLAB’s team adds significant value to every customer interaction.
In addition to its high-quality products and expertise, ESSLAB provides exceptional logistics support. The company’s IATA-qualified team ensures safe and timely shipping of hazardous materials, following all relevant regulations. With a network of trusted carriers, ESSLAB can deliver products efficiently to customers in the UK, Europe, and the Middle East.
Why choose ESSLAB?
ESSLAB is the preferred choice for laboratories and scientific organizations for several reasons:
- IATA Dangerous Goods Regulations Authorized Shipper: ESSLAB ensures all shipments comply with strict safety standards.
- Flexible Shipping Options: The company partners with specialist freight handlers to provide tailored shipping solutions.
- International Trade Expertise: ESSLAB is accredited by the UK Department for International Trade and has over 20 years of experience in exporting.
- Responsive Customer Service: ESSLAB offers live chat, WhatsApp support, and priority response to inquiries.
- Accredited Quality Management: ESSLAB holds ISO 9001 (Quality Management) and ISO 14001 (Environmental Management) certifications.
- Large UK Stock Availability: ESSLAB maintains a large stock of chromatography consumables for quick and reliable delivery.
With its focus on customer satisfaction, high-quality products, and efficient service, ESSLAB is proud to be a trusted partner for laboratories, research institutions, and industries around the world. Whether you need reference materials, precision instruments, or expert logistics support, ESSLAB has the expertise and service to meet your needs.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.