In a recent study published in the journal Signal Transduction and Targeted Therapy, researchers from Australia investigated the potential of small molecule inhibitors, specifically GSK126 (short for ) and Taz (short for tazemetostat), which target the EZH2 (short for Enhancer of zeste homolog 2) methyltransferase protein to stimulate β-cell regeneration in type 1 diabetes (T1D). They found that stimulation with EZH2 inhibitors holds promise for promoting the regeneration of β-like cells from pancreatic ductal progenitor cells, providing a potential avenue for developing novel therapies for T1D.
 Study: EZH2 inhibitors promote β-like cell regeneration in young and adult type 1 diabetes donors. Image Credit: Monkey Business Images / Shutterstock
Study: EZH2 inhibitors promote β-like cell regeneration in young and adult type 1 diabetes donors. Image Credit: Monkey Business Images / Shutterstock
Background
T1D is a chronic condition affecting 400 million people globally, in which the host's immune system destroys β-cells in the pancreas. As these cells synthesize, store, and release insulin in the pancreas, their destruction results in dysregulation of blood glucose levels. Although blood glucose may be managed with pharmacotherapy, none of the current pharmaceutical treatments address the decline in β-cells. While the restoration of β-cell mass through transplantation is clinically effective, the approach is limited by donor shortages and immunosuppression-related risks, emphasizing the critical need for developing innovative therapies.
The existence of pancreatic ductal progenitors for β-cell generation is debated in the field, with conflicting results from observational studies, pancreatic injury models, and lineage tracing experiments. However, recent studies suggest the potential for pancreatic ductal progenitors to differentiate into adult β-cells. Additionally, evidence from a case report suggests that GSK126 (an FDA-approved EZH2 inhibitor) could partially restore insulin function by enabling the conversion of exocrine cells from a T1D donor into β-like cells. Expanding these findings, researchers in the present study characterized the potential of GSK126 and Taz (another EZH2 inhibitor) to restore key β-cell-like function in isolated exocrine cells.
About the study
Using molecular modeling, the structural effects of binding of GSK126 and Taz to EZH2 methyltransferase were analyzed. Ex vivo exocrine tissues (islet, acinar, and ductal) were isolated from three brain-dead donors of varying age and diabetic status: a juvenile with T1D, an adult with T1D, and a healthy non-diabetic adult. The tissues were then stimulated with 10 μM GSK126 or 1 μM Taz for 48 hours. Transcriptomic profiling of the tissues was done using ribonucleic acid (RNA) sequencing and quantitative reverse transcription polymerase chain reaction (qRT-PCR). Chromatin immunoprecipitation (ChIP) experiments and immunofluorescence staining (microscopy) were performed in addition. Further, the researchers developed a glucose-stimulated insulin secretion (GSIS) assay to evaluate the regenerative capacity of diabetic exocrine cells under basal insulin and hyperglycemic conditions.
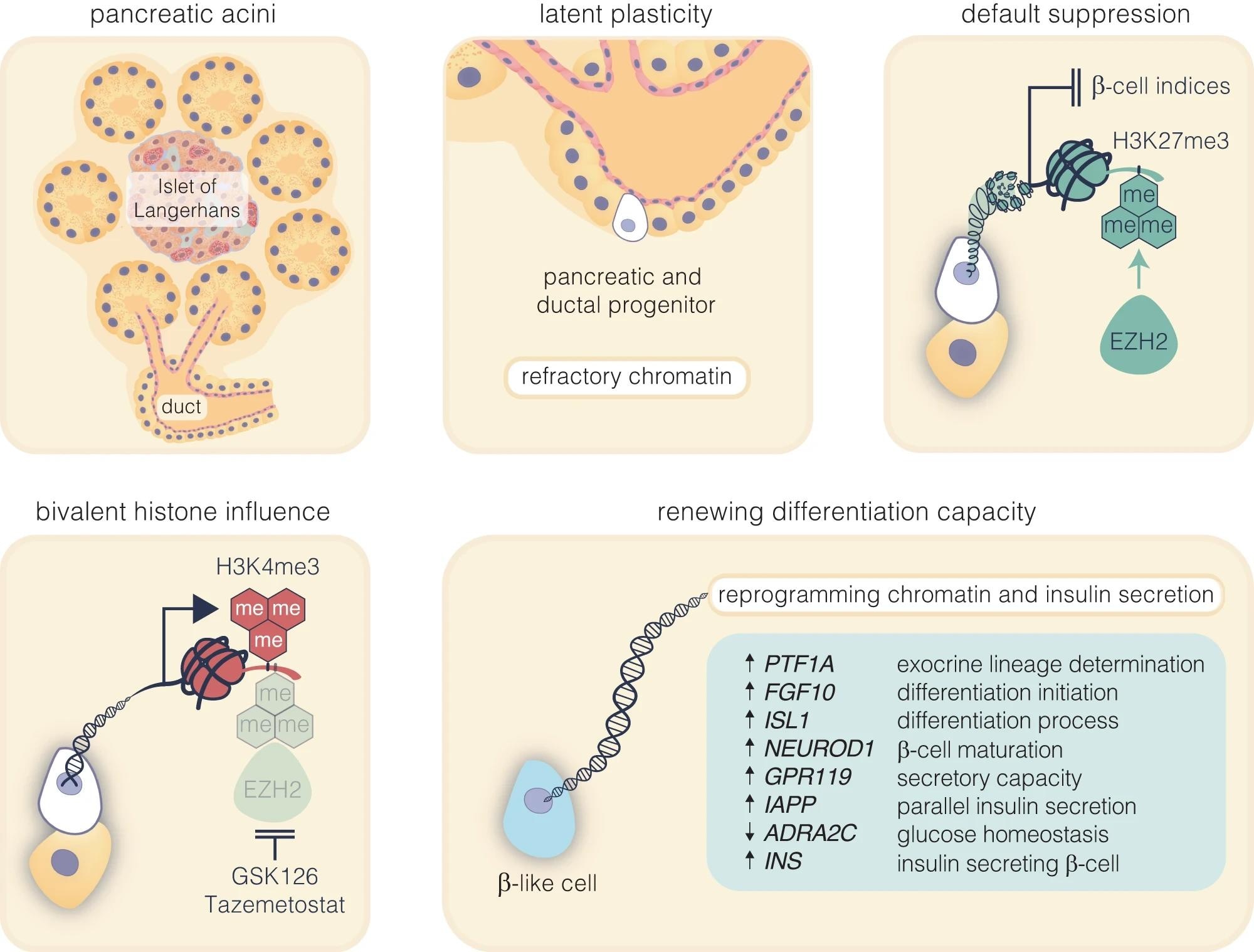 Pharmacological inhibition of EZH2 catalyses pancreatic progenitor activation and β-cell maturation. The schematic outlines the progression from pancreatic multipotent progenitors to mature insulin-secreting β-cells, highlighting the regulatory target of EZH2 inhibitors, GSK126 and Tazemetostat. These progenitors, originating in the Islets of Langerhans’ pancreatic ducts, are maintained in a multipotent state post-development associated with EZH2-mediated suppression with H3K27me3 content enriched on endocrine genes. Reducing H3K27me3 levels shifts bivalent H3K4me3 mark on these progenitors towards the endocrine lineage, marked by PTF1A activation and primes these cells for β-cell differentiation. While FGF10 signalling stabilises this progenitor state, ISL1 and NEUROD1 influence endocrine commitment that support β-cell maturation. Upregulation of GPR119 and IAPP, along with the downregulation of ADRA2C, weakens inhibitory signals, facilitating glucose-stimulated insulin secretion
Pharmacological inhibition of EZH2 catalyses pancreatic progenitor activation and β-cell maturation. The schematic outlines the progression from pancreatic multipotent progenitors to mature insulin-secreting β-cells, highlighting the regulatory target of EZH2 inhibitors, GSK126 and Tazemetostat. These progenitors, originating in the Islets of Langerhans’ pancreatic ducts, are maintained in a multipotent state post-development associated with EZH2-mediated suppression with H3K27me3 content enriched on endocrine genes. Reducing H3K27me3 levels shifts bivalent H3K4me3 mark on these progenitors towards the endocrine lineage, marked by PTF1A activation and primes these cells for β-cell differentiation. While FGF10 signalling stabilises this progenitor state, ISL1 and NEUROD1 influence endocrine commitment that support β-cell maturation. Upregulation of GPR119 and IAPP, along with the downregulation of ADRA2C, weakens inhibitory signals, facilitating glucose-stimulated insulin secretion
Results and discussion
In the modeling studies, Taz was found to show greater binding affinity at the EZH2 catalytic domain than GSK126. Transcriptomic profiling and PCR studies suggest that GSK126 and Taz influence the expression of endocrine markers as well as genes relevant to exocrine hormone regulation and glucose metabolism. Following EZH2 inhibition, refractory H3K27me3 (short for trimethylation of histone H3 at lysine 27) content of endocrine genes was found to be reduced in exocrine tissues. Immunofluorescence staining showed that EZH2-inhibited ductal cells positive for cytokeratin 19 (CK19+ve) could produce insulin. Additionally, stimulated exocrine cells could secrete insulin in a glucose-responsive manner, indicating that glucose homeostasis and mature β-cell activity were functional in the exocrine tissues.
Human pancreatic ductal epithelial cells treated with EZH2 inhibitors GSK126 and Taz exhibited diminished H3K27me3 content. Modest persistence of transcriptional changes was observed even after returning to drug-free conditions at 96 hours. Stimulation of these cells with EZH2 inhibitors did not significantly alter the number of CK19/INS-positive cells. Insulin secretion was elevated during drug stimulation and, although reduced after drug removal, remained higher than the control during glucose stimulation, suggesting a lasting impact on insulin secretion.
The study builds upon previous research and showcases an epigenetics-mediated approach to reprogramming terminally differentiated exocrine cells into insulin-producing β-like cells. It shows, for the first time, the influence of Taz on insulin expression from exocrine ductal cells derived from a diabetic pancreas.
However, the study is limited by its small sample size and is only the second case study of progenitor capacity reinstated in a child with T1D. Additionally, not all ductal cells may undergo β-cell transition, and the conversion efficiency could potentially be enhanced through improved techniques or surgical resection of ductal cells from the pancreas.
Conclusion
In conclusion, the present study's findings imply that the potential to reinstate regenerative β-cell capacity from pancreatic ductal cells may be linked to default suppression. Targeting refractory chromatin by inhibiting EZH2-dependent silencing may help overcome regenerative barriers and restore insulin-producing β-cell-like function in T1D. The findings warrant further research while opening new avenues for therapeutic intervention for patients with T1D.