Granulocyte-macrophage colony-stimulating factor (GM-CSF), also known as Colony Stimulating Factor 2 (granulocyte-macrophage), is a cytokine originally identified for its role in promoting the formation of granulocyte and macrophage colonies from myeloid progenitor cells. It is produced by macrophages, T cells, mast cells, endothelial cells, and fibroblasts. ACROBiosystems' human GMP GM-CSF proteins are produced and quality-controlled in a GMP-compliant facility.
Features
- Designed in accordance with ISO 9001:2015 and ISO 13485:2016 standards
- Produced and quality-control tested within a facility compliant with Good Manufacturing Practices (GMP)
- Utilizes materials free from animal-derived components
- Free of Beta-lactam materials
- Ensures uniformity across production batches
- Subjected to rigorous quality control assays
Source
The GMP Human GM-CSF Protein (GMP-GMFH28) is produced using human 293 cells (HEK293) as the expression system. It encompasses amino acids Ala 18 - Glu 144 (Accession # P04141) of the GM-CSF protein.
Predicted N-terminus: Ala 18.
Molecular characterization

Image Credit: ACROBiosystems
There is no “tag"” on this protein.
The protein's estimated molecular weight is 14.5 kDa. When measured against the Star Ribbon Pre-stained Protein Marker under reducing (R) conditions (SDS-PAGE), the protein migrates as 25 kDa ± 3 kDa as a result of glycosylation.
Endotoxin
Contains less than 10 EU/mg, as determined by the Limulus Amebocyte Lysate (LAL) assay.
Host cell protein
Less than 0.5 ng/µg of protein, as measured by ELISA.
Host cell DNA
Less than 0.02 ng/μg of protein, as quantified by qPCR.
Sterility
Sterility was confirmed using the membrane filtration method outlined in CP<1101>, USP<71>, and Eur. Ph. 2.6.1.
Mycoplasma
Determined to be negative.
Purity
Greater than 95% purity, as assessed by SDS-PAGE.
Formulation
Provided in a lyophilized form from a 0.22 μm filtered solution in PBS (pH 7.4) containing protectants.
Customized product forms or formulations are available upon request.
Shipping
This product is delivered and transported with blue ice.
Storage
Upon delivery, store immediately at -20 °C or lower for extended preservation.
Avoid repeated freeze-thaw cycles.
The product maintains stability under the following storage conditions:
- -20 °C to -70 °C for 5 years in its lyophilized state.
- -70 °C for 12 months under sterile conditions after reconstitution.
ACRO quality management system
- QMS (ISO, GMP)
- Quality Advantages
- Quality Control Process
SDS-PAGE
GMP Human GM-CSF Protein was analyzed using SDS-PAGE under both reducing (R) and non-reducing (NR) conditions. Visualization was achieved through Coomassie Blue staining. The protein exhibited a purity exceeding 95%, as determined alongside a Star Ribbon Pre-Stained Protein Marker.
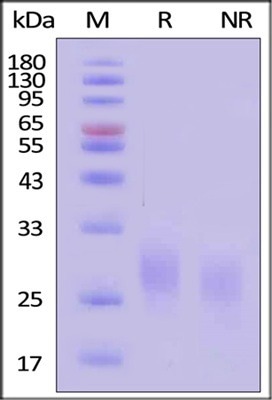
Image Credit: ACROBiosystems
Bioactivity-Bioactivity CELL BASE
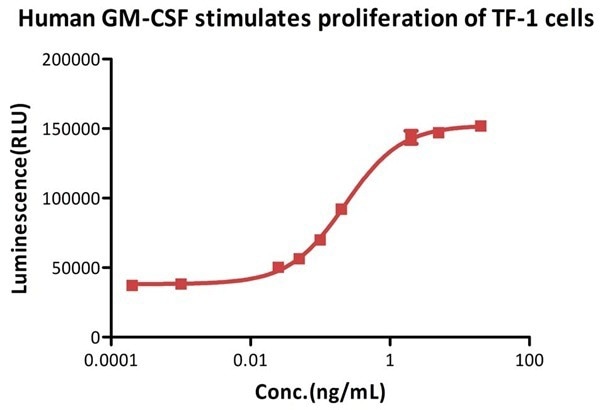
Image Credit: ACROBiosystems
GMP Human GM-CSF Protein (Cat. No. GMP-GMFH28) promotes the proliferation of TF-1 cells. The specific activity is determined to be >5.00 × 106 IU/mg. This activity is calibrated against the World Health Organization (WHO) International Standard for human GM-CSF (NIBSC code: 88/646) and is verified through quality control (QC) testing.
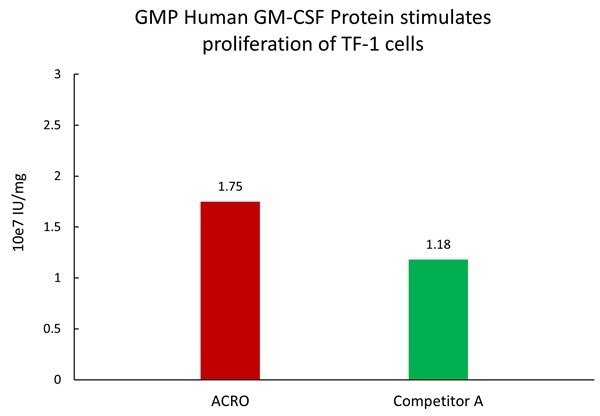
Image Credit: ACROBiosystems
The GMP Human GM-CSF Protein, with Cat. No. GMP-GMFH28, demonstrated superior activity in comparison to other commercially available products.
Bioactivity-Stability
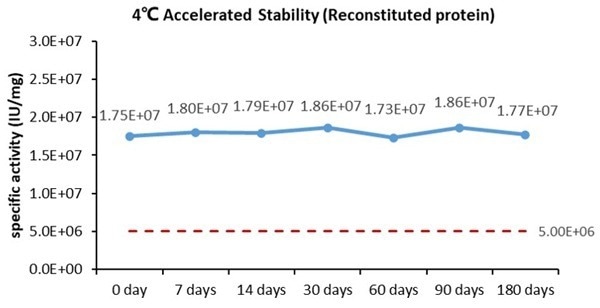
Image Credit: ACROBiosystems
Cell-based assays demonstrate that GMP Human GM-CSF Protein (Cat. No. GMP-GMFH28) maintains its activity when stored at 4 °C for a duration of 180 days.
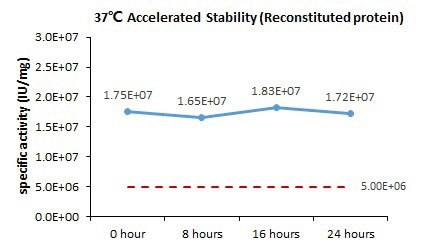
Image Credit: ACROBiosystems
Cell-based assays indicate that GMP Human GM-CSF Protein (Cat. No. GMP-GMFH28) retains its activity after being stored at 37 °C for 24 hours.
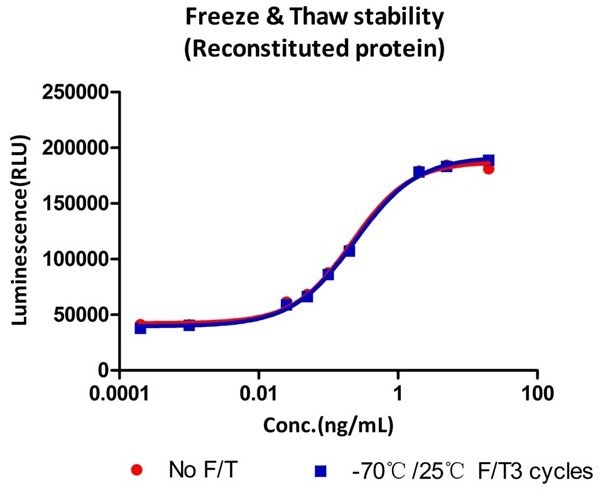
Image Credit: ACROBiosystems
Cell-based assays confirm that GMP Human GM-CSF Protein (Cat. No. GMP-GMFH28) remains active following three freeze-thaw cycles.
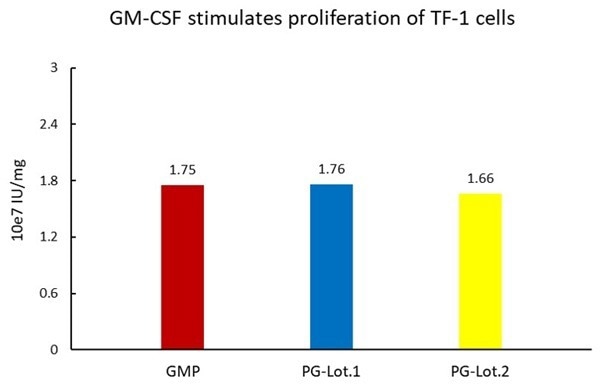
Image Credit: ACROBiosystems
Cell-based assays demonstrate consistent biological activity between batches of ACROBiosystems' GMP and PG GM-CSF.