This article is based on a poster originally authored by C. Yost, H. Wagner, N. Ellis, A. Kauffmann, J. E. Wilson, E. De Vol, and J. Stradinger.
The CDC National Diabetes Statistics Report indicates that in 2021, more than 38 million individuals (all ages) in the United States had diabetes, equivalent to 11.6 % of the population.1 Of this number, 29.7 million had been diagnosed, while approximately 8.7 million remain undiagnosed. Specifically for those 18 years and older, 22.8 % were undiagnosed for diabetes.1
In 2008, the American Diabetes Association organized the International Expert Committee, the European Association for the Study of Diabetes, and the International Diabetes Federation. These organizations collaborated to develop HbA1c-based diagnostic criteria for diabetes.2
Once diabetes is diagnosed, HbA1c levels are tracked to optimize treatment and analyze its effectiveness over time.3 Maintaining optimal HbA1c levels reduces the likelihood of significant complications associated with diabetes, such as cardiovascular diseases, kidney failure, and neuropathy.4
Measuring HbA1c via point-of-care testing (POCT) delivers fast test results during clinical visits. This convenience may also extend access to disadvantaged populations through availability of community testing of HbA1c, as stated by Gourley et al. Siemens-Healthineers provides HbA1c POCT on both the Atellica DCA Analyzer and the DCA Vantage platforms, delivering hemoglobin A1c concentration measurements recommended for tracking long-term diabetes treatment.
To support standardization in HbA1c measurement techniques, the European Reference Laboratory for Glycohemoglobin (ERL) provides value-assigned samples by utilizing multiple reference methods based on distinct measurement principles, all aligned with IFCC secondary reference material.17
HbA1c is created through the non-enzymatic glycation of the N-terminus of the β-chain of hemoglobin A. The HbA1c level represents average glucose concentration over the previous period (about 8–12 weeks, according to the individual), providing better indication of long-term glycemic control than blood or urinary glucose determinations.5
Research has demonstrated that long-term control of HbA1c levels can lower the risk of developing or worsening chronic diabetes-related complications.6,7 Since HbA1c levels are proportional to blood glucose levels over a period of about 2–3 months, HbA1c is recognized as an indicator of average daily blood glucose concentration during that time.8,11
The analytical performance of the Atellica DCA Analyzer and DCA Vantage platforms for HbA1c testing was assessed by measuring a set of ERL value-assigned samples on both platforms. Each platform’s results were then compared individually with the ERL assigned values to evaluate precision and support standardization across POCT methods.
Figure 1. The Atellica DCA Analyzer. Image Credit: Siemens Healthineers
Principles of the procedure
The Atellica DCA Analyzer and DCA Vantage platforms, using the HbA1c Dx and DCA Systems Hemoglobin A1c reagent kits, automatically measure and determine HbA1c through an inhibition of latex agglutination assay.12,13 The concentrations of both hemoglobin A1c and total hemoglobin are measured, and the ratio is reported.10,14
Total hemoglobin is measured by using potassium ferricyanide to oxidize hemoglobin in the sample to methemoglobin. The methemoglobin subsequently complexes with thiocyanate to form thiocyan-methemoglobin, a colored species that is measured. The extent of color development at 531 nm is proportional to the total hemoglobin concentration in the sample.
To measure HbA1c specifically, an inhibition of latex agglutination assay is employed. A synthetic polymer containing multiple copies of the immunoreactive portion of HbA1c serves as an agglutinator, causing agglutination of latex coated with HbA1c-specific mouse monoclonal antibody.
This agglutination reaction increases light scattering, measured as a rise in absorbance at 531 nm. HbA1c in whole blood specimens competes for the limited number of antibody latex binding sites, inhibiting agglutination and decreasing light scattering.
The reduced scattering is measured as a decrease in absorbance at 531 nm. A calibration curve of absorbance versus HbA1c concentration is then used to quantify HbA1c concentration.
%HbA1c = [HbA1c] / [Total hemoglobin] ×100
HbA1c mmol/mol = [HbA1c mmol] / [Total hemoglobin mol]
The Atellica DCA Analyzer HbA1c Reagent Cartridge contains all reagents for performing these measurements. The Atellica DCA Analyzer performs all measurements and calculations automatically, with the screen displaying the HbA1c concentration upon test completion. Values are in % HbA1c NGSP and, where indicated in parentheses, as mmol/mol HbA1c IFCC.
Materials and methods
Method comparisons (CLSI EP09c-ED3)
- Method comparison (MC) studies compared the DCA Vantage platforms HbA1c Cartridge’s (CLIA- waived) and Atellica DCA HbA1c Dx (outside the United States) assay’s performance against ERL value-assigned samples.
- Samples (N=40, value-assigned by ERL) were tested in duplicate on each analyzer. Value-assigned samples were evaluated using both IFCC units (mmol/mol) and NGSP units (%), which were derived from the former.
- Statistical analysis of DCA Vantage HbA1c vs ERL and Atellica DCA HbA1c vs ERL (multilevel means as outcomes linear least-squares) and DCA Vantage vs Atellica DCA HbA1c (Weighted Deming) was performed and coefficients of determination (pseudo-R2) calculated. In addition, a method comparison of the DCA Vantage versus Atellica DCA HbA1c was conducted (Weighted Deming) using the Replicate 1 results and subsequently repeated with Replicate 2 results.
Results
The performance of the assay is represented by the following results. The Atellica DCA Analyzer and DCA Vantage platforms demonstrated comparable performance, with results exhibiting close alignment with each other and with the ERL-assigned reference values across the 40 tested samples.
Table 1. Summary of HbA1c (%) testing on Comparative Devices. Source: Siemens Healthineers
Test
Method |
Reference
Method |
Regression
Analysis model |
N |
Slope |
Intercept |
R2 |
DCA Vantage
systems |
ERL
Assigned |
ML means as outcomes
least squares* |
40 paired
replicates |
0.9726 |
0.1766 |
0.9999 |
Atellica DCA
Analyzer |
ERL Assigned |
ML means as outcomes
least squares* |
40 paired
replicates |
0.9970 |
0.0702 |
0.9983 |
Test
Method 1 |
Test
Method 2 |
Regression
Analysis model |
N |
Slope |
Intercept |
R2 |
Atellica DCA
Analyzer |
DCA Vantage
systems |
Weighted
Deming |
40 paired
replicates |
1.0251 |
-0.1103 |
0.9928 |
*Multilevel (ML) means-as-outcomes linear least squares model.
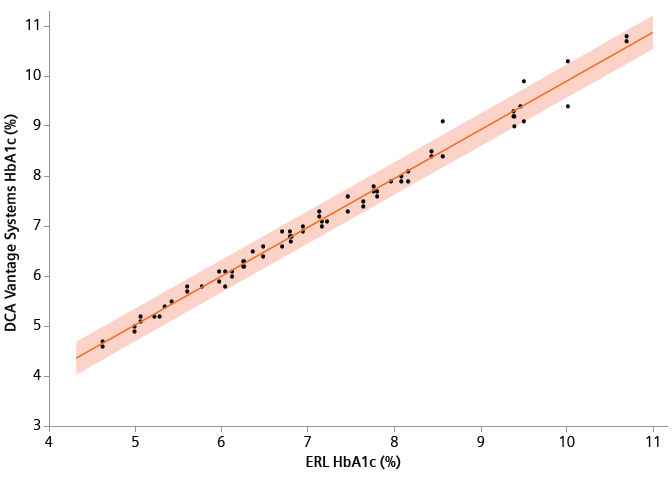
Figure 1. Comparison of the DCA Vantage systems to ERL HbA1c methods (orange, band is a 95% prediction interval). Samples ranged from 4.62% HbA1c to 10.69% HbA1c (27.00 to 93.34 mmol/mol). The comparison of the DCA Vantage systems to ERL methods of testing HbA1c level yielded y = 0.9726 x + 0.1766% HbA1c (y = 0.9726 x+ 1.3188 mmol/mol, with R2=0.9999). Image Credit: Siemens Healthineers
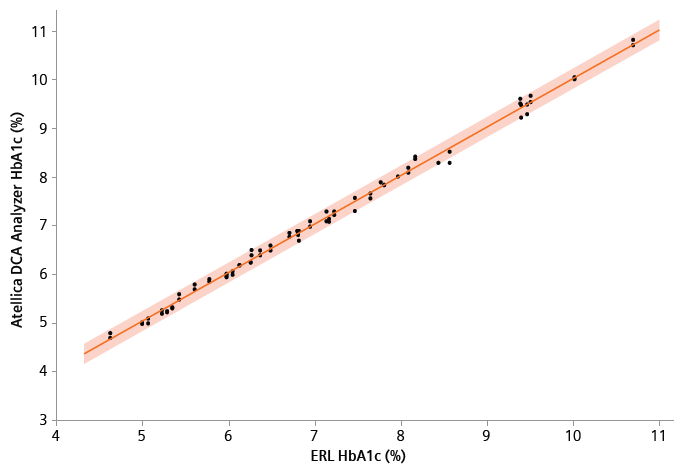
Figure 2. Comparison of the Atellica DCA Analyzer to ERL HbA1c methods (orange, band is a 95% prediction interval). Samples ranged from 4.62% HbA1c to 10.69% HbA1c (27.00 to 93.34 mmol/mol). The comparison of the Atellica DCA Analyzer to ERL method of testing HbA1c levels yielded y = 0.9970 x + 0.0702% (y = 0.9973 x+ 0.6967 mmol/mol, with R2=0.9983). Image Credit: Siemens Healthineers
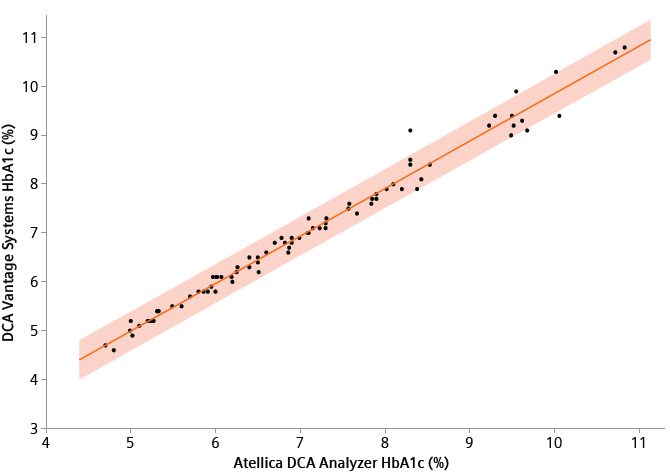
Figure 3. Comparison of the DCA Vantage systems to Atellica DCA Analyzer HbA1c methods (orange, band is a 95% prediction interval). Samples ranged from 4.62% HbA1c to 10.69% HbA1c (27.00 to 93.34 mmol/mol). The comparison of the Atellica DCA Analyzer to DCA Vantage systems of testing HbA1c levels yielded y = 1.0251 x + -0.1103% (y = 0.9756 x+ 0.6018 mmol/mol, with R2=0.9929). Image Credit: Siemens Healthineers
Medical decision levels
According to the American Diabetes Association, clinical decisions are made at 5.7%, signifying the onset of pre-diabetes, and at 6.5% or higher, diagnosing type 2 diabetes in most individuals.15 Relative differences [100 x (assay – (ERL-assigned)) / (ERL-assigned)] at these clinical decision levels comparing the ERL method results against the DCA Vantage platforms and Atellica DCA Analyzer HbA1c methods were calculated: at 5.7% (ranges: 5.4% to 6.0% and 5.6% to 6.0% respectively) and 6.5% (ranges: 6.2% to 6.8% and 6.4% to 6.8%, respectively).
Table 2. Summary of Decision Level Comparability. Source: Siemens Healthineers
| Methods |
HbA1c (%) |
Relative Difference |
| Atellica DCA Analyzer vs ERL |
5.7 |
0.7 % |
| 6.5 |
0.6 % |
| 8.0 |
0.6 % |
| 12.0 |
0.6 % |
| DCA Vantage systems vs ERL |
5.7 |
0.8 % |
| 6.5 |
0.2 % |
| 8.0 |
-0.5 % |
| 12.0 |
-1.6 % |
Atellica DCA Analyzer vs
DCA Vantage systems |
5.7 |
0.1 % |
| 6.5 |
0.5 % |
| 8.0 |
1.1 % |
| 12.0 |
2.0 % |
Conclusions
This study’s findings show that the HbA1c measurements from the Atellica DCA Analyzer and the DCA Vantage platforms correlate strongly with those from the European Reference Laboratory (ERL), as demonstrated by R² values exceeding 0.99 in method comparisons. These results validate the analytical strength and clinical dependability of both Siemens Healthineers’ point-of-care systems in HbA1c measurement across a clinically relevant concentration range.
In addition, the strong agreement between the two platforms (R² = 0.9928), along with their consistent performance at critical medical decision thresholds (5.7 %, 6.5 %, 8.0 %, and 12.0 % HbA1c), support their interchangeability for monitoring diabetes in diverse healthcare environments. This high degree of agreement with an international reference standard underscores their value in delivering timely and evidence-based diabetes management.
Acknowledgments
Poster produced from materials originally authored by C. Yost, H. Wagner, N. Ellis, A. Kauffmann, J. E. Wilson, E. De Vol, and J. Stradinger at Siemens Healthcare Diagnostics Inc., Tarrytown, NY, U.S.
References and further reading:
- CDC (2024). National diabetes statistics report. (online) Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/diabetes/php/data-research/index.html.
- International Expert Committee (2009). International Expert Committee Report on the Role of the A1C Assay in the Diagnosis of Diabetes. Diabetes Care, (online) 32(7), pp.1327–1334. https://doi.org/10.2337/dc09-9033.
- ElSayed, N.A., et al. (2024). 7. Diabetes Technology: Standards of Care in Diabetes - 2025. Diabetes Care, (online) 48(Supplement_1), pp.S146–S166. https://doi.org/10.2337/dc25-s007.
- Li, S., et al. (2019). Visit-to-Visit HbA1c Variability Is Associated With Cardiovascular Disease and Microvascular Complications in Patients With Newly Diagnosed Type 2 Diabetes. Diabetes Care, 43(2), pp.426–432. https://doi.org/10.2337/dc19-0823.
- Goldstein, D.E., et al. (2004). Tests of Glycemia in Diabetes. Diabetes Care, (online) 27(7), pp.1761–1773. https://doi.org/10.2337/diacare.27.7.1761.
- The Diabetes Control and Complications Trial Research Group (1993). The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. New England Journal of Medicine, (online) 329(14), pp.977–986. https://doi.org/10.1056/nejm199309303291401.
- Rohlfing, C.L., et al. (2002). Defining the Relationship Between Plasma Glucose and HbA1c: Analysis of glucose profiles and HbA1c in the Diabetes Control and Complications Trial. Diabetes Care, 25(2), pp.275–278. https://doi.org/10.2337/diacare.25.2.275.
- American Diabetes Association (2017). 6. Glycemic Targets: Standards of Medical Care in Diabetes - 2018. Diabetes Care, (online) 41(Supplement 1), pp.S55–S64. https://doi.org/10.2337/dc18-s006.
- Kilpatrick, E.S., Bloomgarden, Z.T. and Zimmet, P.Z. (2009). International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes: response to the International Expert Committee. Diabetes care, (online) 32(12), pp.e159; author reply e160. https://doi.org/10.2337/dc09-1231.
- Sacks, D.B. (2012). Measurement of Hemoglobin A1c: A new twist on the path to harmony. Diabetes Care, (online) 35(12), pp.2674–2680. https://doi.org/10.2337/dc12-1348.
- Zhang, X., et al. (2010). A1C level and future risk of diabetes: a systematic review. Diabetes Care, (online) 33(7), pp.1665–1673. https://doi.org/10.2337/dc09-1939.
- Atellica DCA HbA1cDx Assay Instructions for Use [package insert]. Tarrytown, NY: Siemens Healthineers; 2023. Document Library - Siemens Healthineers
- DCA Systems Hemoglobin A1c Reagent Kit Instructions for Use [package insert]. Tarrytown, NY: Siemens Healthineers; 2017. DCA® HbA1c Reagent Kit* for DCA Vantage® Analyzer - Siemens Healthineers
- Sacks, D.B. (2012). Hemoglobin A1cin Diabetes: Panacea or Pointless? Diabetes, 62(1), pp.41–43. https://doi.org/10.2337/db12-1485.
- CDC (2024). Testing for Diabetes and Prediabetes: A1C. (online) Diabetes. Available at: https://www.cdc.gov/diabetes/diabetes-testing/prediabetes-a1c-test.html.
- Gourlay, A., C. Sutherland, and A. Radley, Point-of-care testing of HbA1c levels in community settings for people with established diabetes or people at risk of developing type 2 diabetes: a systematic review and meta-analysis protocol. BMJ Open, 2023. 13(5): p. e072882.
- IFCC (2024). IFCC HbA1c Standardization Program. (online) International Federation of Clinical Chemistry and Laboratory Medicine. Available at: https://www.ifcchba1c.org/.
About Siemens Healthineers
Siemens Healthineers develops innovations that support better patient outcomes with greater efficiencies, giving providers the confidence they need to meet the clinical, operational and financial challenges of a changing healthcare landscape. As a global leader in medical imaging, laboratory diagnostics, and healthcare information technology, we have a keen understanding of the entire patient care continuum - from prevention and early detection to diagnosis and treatment.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last Updated: Oct 22, 2025