Introduction
hCG and pregnancy
The developing placenta secretes the glycoprotein hormone hCG, human chorionic gonadotropin, soon after fertilization. In normal pregnancy, this hormone can be detected in serum in just seven days after conception.
Concentrations of urine hCG are about one half (or less than one half) of the corresponding concentrations of serum hCG. As a result, the hCG hormone can be detected in urine in just 14 days following conception (about 28 days since the last menstrual cycle).
About every two days, the hCG doubles in concentration until it peaks at about 8 – 10 weeks after the last menstrual cycle. However, the hCG level decreases over the next 10 or more weeks. The hormone appears soon after conception and its rapid rise in concentration thereafter during early gestational growth makes this an excellent and reliable marker for the early detection of pregnancy.
The CLINITEST hCG test
The CLINITEST hCG test employs an easy-to-use cassette that is utilized with the CLINITEK Status family of analyzers. After mounting the cassette on a test table, a urine sample is added to the sample well and the cassette is then automatically drawn into the analyzer. The analyzer reports qualitative hCG results as negative, positive or borderline:
- When the urine hCG level is ≥ 25 mIU/mL, it indicates a positive result
- When the urine hCG level is ≤ 5 mIU/mL, it indicates a negative result
- When the urine hCG level is between 5 and 25 mIU/mL, it indicates a borderline result
However, borderline hCG results are believed to be indeterminate for pregnancy and the test has to be repeated within 48 to 72 hours. If negative test results are obtained in women suspected to be pregnant, a re-test should be performed on a urine sample acquired 48 to 72 hours later.
In order to exclude pregnancy with the highest level of certainty, it is important to perform repeat testing on a sample acquired 48 to 72 hours later following an initial negative result. As with any kind of diagnostic test, a single CLINITEST hCG test result should not be used to make a clinical diagnosis.
If non-pregnancy is suspected despite obtaining a positive test result, the test should be repeated with another urine sample acquired 48 hours later as per the standard clinical and laboratory practice. In acute situations, a quantitative serum hCG test is recommended to confirm the result.
A general rule is that any CLINITEST hCG test result that does not fit a patient’s clinical picture should be immediately followed up with repeat later testing on a fresh sample and a quantitative serum hCG test should then be performed.
False-negative, false-positive and borderline hCG results
Even when a proper method is used and testing protocols are accurately followed, inaccurate (false-positive and false-negative) pregnancy test results may still occur. A small number of false-positive results are produced by all qualitative pregnancy tests.
Other than pregnancy, borderline hCG levels can also be caused by a number of medical conditions such as certain non-trophoblastic neoplasms and trophoblastic disease.
While borderline CLINITEST hCG results are not regarded as diagnostic, the lack of procedural error may indicate to clinicians that additional investigation is required, especially when test results do not fit a patient’s clinical picture. Summarized in Table 1 are causative factors (excluding procedural error) that contribute to false-positive, false-negative and borderline hCG results with the CLINITEST hCG test.
The advantages of analyzer-read CLINITEST hCG testing
Analyzer-read chromatographic immunoassay tests are increasingly replacing qualitative visual ‘dip and read’ pregnancy tests. These tests are performed to detect the presence of hCG in urine samples. Compared to conventional dipstick methodology and reporting, accurate and reliable analyzer read pregnancy test results provide considerable benefits.
The risk of error is also considerably reduced as no manual documentation or visual interpretation is needed.
Pregnancy test analyzers that are coupled to a Hospital Information System (HIS) or Laboratory Information System (LIS) (for instance, the CLINITEK Status Connect System) automatically provide the hCG results, which can be acted upon instantly; these analyzers also enable the trend towards healthcare provision and testing in ambulatory, decentralized settings.
Table 1. Potential causes of false-negative, false-positive and borderline hCG results.
|
Potential causes of a false-negative result
|
Potential causes of a false-positive result
|
Potential causes of a borderline result
|
- Very early pregnancy
- Pre-embryo implantation
- Concentrated urines that contain low levels of hCG
- Urine contaminated with proteolytic enzymes
- Degradation in old samples
- Interfering antibodies in the medications of patients on antibody therapies
|
- Undetected early spontaneous abortion (approximately 22% of pregnancies abort spontaneously, without being detected)
- The presence of interfering substances such as microorganisms, drugs, endogenous proteins, erythrocytes, and/or leukocytes
- The detection of low concentrations of hyperglycosylated hCG and beta core fragments (detected very early in pregnancy) that may not be detected by other manufacturers’ tests
|
- Undetected early spontaneous abortion
- Ectopic pregnancy, when hCG levels rise at a significantly slower rate than in normal pregnancy
- Trophoblastic diseases, including a variety of cancers
- Increased levels of hCG in healthy, post-menopausal, non-pregnant women
- hCG passively acquired from a blood transfusion
- hCG levels may still be detected for up to several weeks after miscarriage, delivery, or hCG injections (from IVF treatment)
- Interfering antibodies in the medications of patients on antibody therapies
|
CLINITEST hCG testing on the CLINITEK status family of analyzers is CLIA-waived in the United States
- Transcription errors are eliminated with accurate and reliable analyzer read-and-reported results
- Positive results are reported in just two minutes, while negative results are reported within five minutes
- The analyzer accurately times the reading of results, and confirms that the test has been properly completed
- Patient and operator ID are included in a printed record of results
- CLINITEST hCG testing eliminates hands-on waiting time which improves workflow and increases the time for patient care
- The CLINITEK Status family of analyzers performs automatic quality checks for proper sample flow and adequate sample which helps improve QC. The analyzers create error flags when such conditions are not met
- It is possible to program the CLINITEK Status Connect System analyzers to automatically check for QC results and start a QC lock-out if results fail
- The CLINITEK Status Connect System allows the results to be automatically transmitted to a HIS or LIS for better data capture
Study purpose
In 2012, a new quality control system was launched by Siemens Healthcare Diagnostics to supervise the development of CLINITEST hCG test cassettes. The aim of this analysis was to reiterate the appropriate sensitivity and clinical performance criteria of the CLINITEST hCG test following the development of new measures and processes under which CLINITEST hCG test cassettes are developed and launched.
Reaffirmation of accurate, dependable results ensures that healthcare practitioners can confidently employ the test as a rapid and reliable marker for early detection of pregnancy. The study investigated:
- Rates of false-positive and borderline results with hCG negative clinical samples
- Sensitivity of the test with spiked samples, negative and positive urine samples
- The rate of agreement of positive results with positive clinical samples
Methods
Performance study
At the Siemens facility in Elkhart, Indiana, the performance of the CLINITEST hCG test was evaluated on three lots of newly developed CLINITEST hCG pregnancy test cassettes over a period of two months in July and August 2012.
About 675 hCG positive and hCG negative clinical urine samples (acquired internally as well as from external clinical sites) were tested on three CLINITEK Status analyzers and six CLINITEK Status+ across the three lots, constituting a study involving more than 18,000 individual tests. Among the samples tested, 175 were hCG positive (> 25 mIU/mL) and 500 were hCG negative.

With the help of a standard laboratory pipette, 200 μL of each sample was subsequently applied to each cassette. One replicate of each sample was then run on each analyzer for each CLINITEST hCG cassette test lot. After testing the samples at room temperature on a cassette lot, the same were frozen at -20 °C until required for testing with the subsequent cassette lot.
Testing was carried out in multiple runs, with both positive and negative samples tested in each run. At the beginning of each testing day, Bio-Rad qUAntifyR Plus Urinalysis Control, Level 1 and Level 2, was tested on all analyzers for QC purposes. Validated DCAT data collection software was used to collect test data, and the occurrence rates for positive and borderline results were measured.
Sensitivity study
Using three CLINITEK Status and six CLINITEK Status+ analyzers, clinical sensitivity of the CLINITEST hCG test was evaluated on three lots of newly developed CLINITEST hCG pregnancy test cassettes. From three known non-pregnant individuals, hCG negative urine samples were acquired and these were pooled to achieve sufficient volume.
This was followed by dividing the urine pools into nine different samples and spiking them to hCG concentrations of 0, 2, 5, 10, 15, 20, 25, 50, and 100 mIU/mL. After the spiked sample hCG concentrations were verified, three replicates of each sample were run on each analyzer by means of the cassette test lot.

Using a standard laboratory pipette, 200 μL of each sample was then applied to each cassette. 27 replicates per hCG concentration were run on CLINITEK Status analyzers, and 54 replicates per hCG concentration were run on CLINITEK Status+ analyzers.
Then, using the Bio-Rad Quantify Plus Urinalysis Control, Level 1 and Level 2, QC was carried out at the beginning of testing with each CLINITEST hCG cassette test lot. Validated DCAT data collection software was used to collect the test data, and this is followed by measuring the percent positive results for each spiked sample test.
Table 2. Negative sample testing results.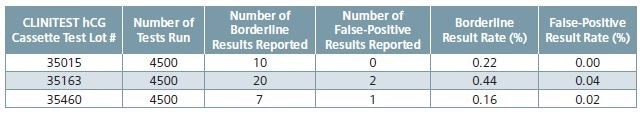
Table 3. Results from spiked sample replicate testing across all analyzers and CLINITEST hCG cassette test lots (N = 54 or N = 27).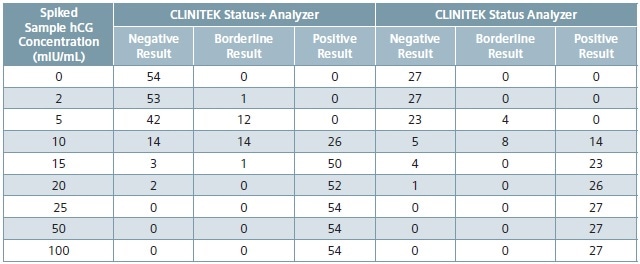
Table 4. Calculated percent positive results by spiked hCG concentration across analyzers and all CLINITEST hCG cassette test lots.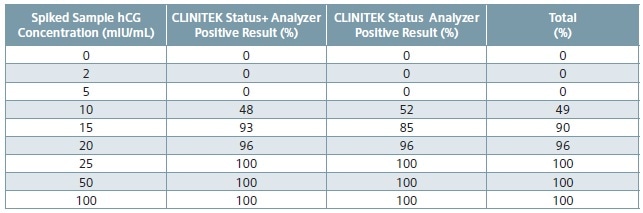
Results
Performance study
Shown in Table 2 are the borderline results reported and the borderline rates measured, from negative testing of hCG sample across three lots of CLINITEST hCG test cassettes. Also shown are the positive results reported and the false-positive rates measured.
The rate of borderline results with negative patient urines ≤ 0.9%1 is one measure of acceptable clinical performance with regards to the CLINITEST hCG test on the CLINITEK Status family of analyzers. This is an internal validation study which reported borderline rates by CLINITEST hCG cassette test lot of 0.16%, 0.22% and 0.44% with negative clinical samples.
Among the 13,500 tests, only three reported a false-positive result with a negative sample indicating a ≤ 0.04% rate across the three CLINITEST hCG cassette test lots.
For each CLINITEST hCG cassette test lot, 175 positive (hCG > 25 mIU/mL) clinical samples were run on all nine CLINITEK Status and CLINITEK Status+ analyzers (a total of 4,725 individual tests). A negative result was returned by only one sample on one CLINITEK Status+ analyzer (with lot #35163); the sample reported positive on all other CLINITEK analyzers.
For positive results with positive hCG samples, the acceptable rate of agreement is ≥ 99.1%.1 In the case of cassette test lot #35163, 99.9% is the rate of agreement for positive results with positive samples; lots #35460 and #35015 showed a rate of agreement for positive results with positive samples of 100.0%.
Sensitivity study
Table 3 shows spiked sample test results across all CLINITEST hCG cassette test lots and across all analyzers, and Table 4 reveals the calculated percent positive results by hCG concentration across all CLINITEST hCG cassette test lots and across all analyzers.
Urinary hCG concentrations of at least 25 mIU/mL (calibrated to the World Health Organization 3rd International Reference Preparation) is detected by the CLINITEST hCG test. Acceptable sensitivity at hCG concentrations of 25, 50 and 100 mIU/mL on the CLINITEK Status family of analyzers is 100% positive results.2
Acceptable sensitivity at a hCG concentration of 0 mIU/mL requires that all samples report negative.2 All sensitivity acceptance criteria were met by the results reported in the current study. At 100, 50, and 25 mIU/mL levels, all the results across all CLINITEST hCG cassette test lots and across all analyzers were positive. At the clinical level of 0 mIU/mL, all samples returned a negative result.
Discussion and conclusions
Study results obtained from negative hCG sample testing showed an overall occurrence of borderline results ≤ 0.44%, which were well within the acceptable test performance criterion of a ≤ 0.9% rate with negative urines.
Among the 13,500 negative urine samples tested (≤ 0.04%), there were three incidences of false-positive results. The rate of agreement of positive results with positive samples was ≥ 99.9%, exceeding the performance acceptance criterion of ≥ 99.1%.
Moreover, the results from this study showed concordance with all sensitivity acceptance criteria. All of the tests on all negative samples (0 mIU/mL) and all of the tests on all positive spiked samples (at 25, 50, and 100 mIU/mL) reported negative and positive results, respectively.

The validation study results, taken collectively, demonstrated clinical performance and sensitivity acceptance criteria of the CLINITEST hCG test, following the introduction of new measures and processes to handle the development of CLINITEST hCG test cassettes.
When accurate and reliable results are available, it gives confidence to healthcare practitioners to use the CLINITEST hCG test as a fast, dependable marker for early diagnosis of pregnancy. The results of the present study support the growing uptake of the CLINITEST hCG test as an alternative means to conventional ‘dip and read’ pregnancy test approaches.
Acknowledgements
Produced from materials originally authored by Amy Zercher, Manager of Verification and Validation, Global Assay Development, Siemens Healthcare Diagnostics.
References
- CLINITEST hCG Pregnancy Test Instructions for Use (IFU). 06878007, 2008-08. See Performance Characteristics: Method Comparison.
- CLINITEST hCG Pregnancy Test Instructions for Use (IFU). 06878007, 2008-08. See Performance Characteristics: Sensitivity.
About Siemens Healthineers Point of Care Diagnostics

Point-of-care solutions are designed to provide immediate, convenient, and easy-to-use diagnostic testing. From the ED to the physician’s office, clinical management decisions can be made immediately and result in improved patient safety, clinical outcomes, and overall patient satisfaction.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.