In comparison to other industries, the medical one must meet higher standards for quality and documentation. Manufacturers of medical products must guarantee consistent high quality standards during production.
Furthermore, the long-term reliability of delivery and compliance with the legal framework must be ensured. Quality control includes the development and production, as well as the packaging of the medical components.
Below are presented some of the recently completed projects in the medical packaging industry that illustrate this point.
Fully Automated Function Testing of Autoinjectors to EN ISO 11608-5
An autoinjector is a medical device which administers liquid medication. The solution offered by ZwickRoell performs the following fully automated tests:
- Removal force of the safety cap
- Activation force and displacement of the autoinjector
- Injector timing
- Evaluation of the administered drug volume, including the last drops with the use of an integrated high-resolution scale
- Effective needle length for injection
- Safety function of the needle guard
Fully-automated solutions range from smart robots that are easy to use and are only used for feeding and removing the injector, to more complex robotic systems, which are used to simultaneously load specimens into multiple testing machines.
Completely automated daily checks can be implemented with complex robotic systems. The results and events can be uploaded automatically into the IT system of the customer, for instance via an OPC interface; the injectors can also be sorted based on specified tolerances.

Image credit: ZwickRoell Gmbh Co. KG
Fully Automated Torsion Testing on Pharmaceutical Bottles
Plastic medicine bottles that contain medicine for children have a childproof seal to make sure that they cannot be opened without adult supervision. A combination of compressive and torsional loading needs to be applied for the cap to be removed. The parameters of these are determined through the testing system.
The testing system contains a small machine with 1 kN nominal force plus a 2 Nm torsion drive that convey the testing of the materials. The roboTest L robotic testing system has a depository for 30 medicine bottles that are processed fully automatically. After the test, the medicine bottles are placed back in the depository.

Image credit: ZwickRoell Gmbh Co. KG
Fully Automated Determination of the Breakaway Force and Glide Force for Syringes, Carpules and Similar Drug-delivery Systems
The following standards are applied to glide force testing: EN ISO 7886-1, ISO 11499 and ISO 11040-4. The most suitable syringe is selected based on the breakaway force and glide force, which make the parameters important. To make sure that a safe drug dose is administered, the forces should not exceed or fall below certain limits.
The viscosity of the drug and the size of the cannula influence the forces. The smart robot solution is used in this testing as well. The robotic gripping arm takes one of the 30 syringes from the depository table, places it in the testing machine and starts the determination of the glide force. After the test, the robot places the syringe back in its original location in the depository.

Image credit: ZwickRoell Gmbh Co. KG
Fully Automated Tests on Pharmaceutical Pens
The reproducibility of results and the minimization of operator influences are the most important requirements when conducting testing of medical products. ZwickRoell has developed automated systems for testing pharmaceutical pens, for instance for insulin, in order to meet these requirements.
The pens are similar to writing pens and are frequently filled with insulin cartridges. Single-use pens are disposed of after the contents of the cartridge are dispensed, whereas reusable pens can be used year after year.
The standard used for quality assurance tests on insulin pens and cartridges is DIN EN ISO 11608 Parts 1 to 3. In terms of the autoinjectors, the fully automated solutions range from simple smart robots used for feeding and removing to robots that operate simultaneously on multiple machines.
Key Benefits of Automation
Industrie 4.0
The autoEdition3 control software regulates the robotic testing system. The basic principles of Industry 4.0, such as decentralized intelligence, simultaneous processes, standardized interfaces and real-time optimization are applied to the testing system. The roboTest testing systems allow ZwickRoell to achieve higher specimen throughput in comparison to traditional, sequentially controlled systems.
Traceability
In the medical and pharmaceutical industries, it is mandatory that complete backward tracing is possible for several years. To meet the requirements for this, ZwickRoell's testXpert III testing software provides the necessary additional functionalities. There is a software option from ZwickRoell that allows complete, tamper-proof documentation of all actions performed in testXpert III.
The user sets the level at which actions are to be logged and explained in accordance with their requirements. In individual cases this may lead to full recording of each change made to a test-relevant parameter, for instance, test speed. Together with the user management already integrated in testXpert III, the recording option provides the ideal tool for fulfilling the requirements from FDA 21 CFR Part 11 (regulation of the US Food and Drug Administration).
Providing easy access to quality characteristics as well as protection from tampering allows manufacturers to both optimize their processes and sustain improvements in product quality for safety-related products.
Automatic Daily Check Tools
In order to regularly check the sensors for force, weight or displacement, daily check tools are used. They recognize systematic measurement errors in the sensors and notify the responsible person immediately. Usually, the process takes place at least every 24 hours.
There is a manual version in which the user engages and monitors the daily check tool; along with it ZwickRoell also offers a fully automated version in which all of the steps are handled via automation. The results are recorded in a log file and can be traced. The system stops when the results are bad and notifies the user.
Qualification Support
A key part of process validation in the medical and pharmaceutical industry is the technical review of individual plant and devices. The qualification is required for ZwickRoell materials testing systems used in the aforementioned industries, because systems are subject to various legal requirements, for instance, the Medical Devices Directive 93/42/EEC or regulations, such as FDA 21 CFR Part 11.
ZwickRoell supports the qualification of materials testing systems in the Design Qualification (DQ), Installation Qualification (IQ), and Operational Qualification (OQ) processes. To do so, it offers a comprehensive, and if requested, individually tailored qualification documentation. ZwickRoell also offers practical performance qualification on site.
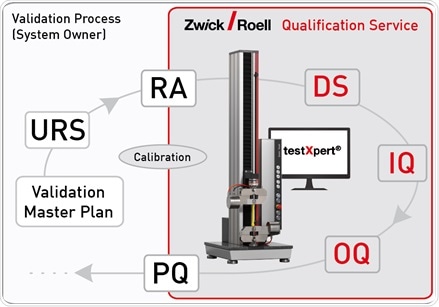
Image credit: ZwickRoell Gmbh Co. KG
OPC Connection
A central computer collects the determined parameters and measured values; a signature from the responsible employee needs to be applied to these. Live data, such as events like “all specimens processed,” can be transmitted directly via the OPC interface.
About ZwickRoell, LP
With over 160 years of expertise in materials and components testing, ZwickRoell delivers intelligent materials testing solutions for the medical industry, including drug delivery testing systems for syringes and cartridges, autoinjectors, and pen injectors. Our qualification services help ensure compliance to your industry’s stringent testing requirements.
Worldwide Presence
ZwickRoell, LP is a member of the ZwickRoell Group, a key player in the global market for materials testing systems. Operating in 56 countries worldwide, the ZwickRoell Group has manufacturing facilities in Germany, the UK, Austria, and China, well as subsidiaries in France, the UK, Spain, the USA, Brazil, Turkey, Singapore, and China.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.