Coronaviruses are a family of enveloped, positive-sense RNA viruses affecting the upper respiratory tract, usually only causing mild illnesses similar to the common cold.
However, in the past two decades, there are three zoonotic pathogens that have arisen and led to severe and, in many cases, fatal outcomes for humans.1 These are severe acute respiratory syndrome coronavirus (SARS-CoV)-1, SARS-CoV-2, and Middle East respiratory syndrome coronavirus (MERS-CoV).
As of 22 July 2020, the current coronavirus SARS-CoV-2 pandemic death toll has exceeded 600,000 and has infected over 15 million people across 188 countries.2
In late 2019, the novel virus was transmitted to humans, and as of July 2020, no approved therapeutic interventions have been made available.
In order to reduce the spread of SARS-CoV-2, millions of people have been advised to stay at home in an attempt to limit the number of deaths. However, effective treatments and vaccines are necessary in order to successfully fight the virus.1
SARS-CoV-2 Treatment Targets
The spike (S) glycoprotein found on the surface of the outer membrane envelope of coronaviruses is a promising therapeutic target. A trimeric complex with two subunits, S1 and S2, divided by a protease cleavage site, is formed from the S protein.
The S1 subunit has a receptor-binding domain (RBD), which is responsible for interacting with a receptor protein on the host cell surface. This receptor protein is dipeptidyl peptidase 4 (DPP4) for MERS-CoV, and angiotensin-converting enzyme 2 (ACE2) for SARS-CoV-1 and SARS-CoV-2.1 After this, the S2 subunit is responsible for fusion of the host membranes and the viral envelope to enable the virus particle to pass into the host cell.4
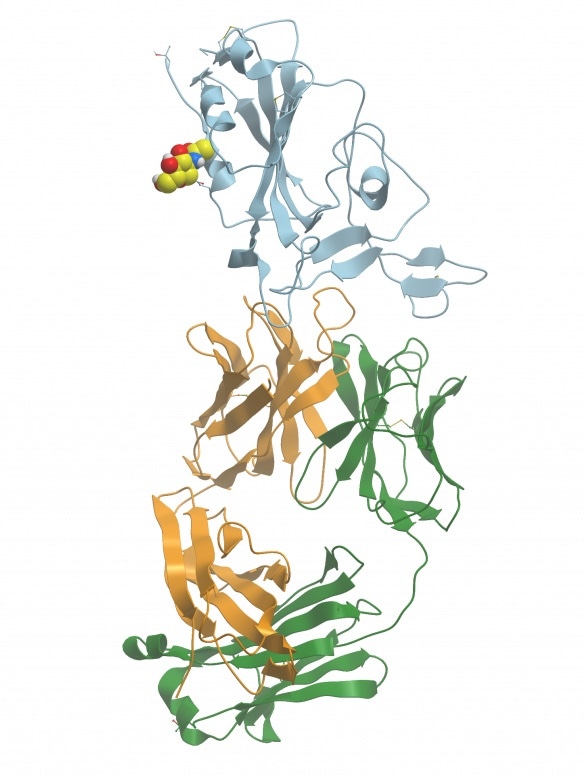
Figure 1. Crystal structure of SARS-CoV-2 antibody with RBD. PDB ID: 7BWJ(Zhang et al 2020). Image generated with Molsoft ICM Browser. Image Credit: Jackson ImmunoResearch Laboratories, Inc.
Drugs that bind to the RBD on the S1 subunit of the S protein inhibit binding to the ACE2 receptor and can prevent fusion of the virus envelope with the host cell, resulting in neutralization of the coronavirus.
Using cryoelectron microscopy, it has been shown that the conformation of the RBD changes frequently. There have been two key configurations characterized; the up conformation, for which the RBD is accessible and can engage with the host-cell receptor readily, and the down conformation, for which the RBD is hidden by the top of the S2 subunit and binding is prevented. Hence, drug binding is theorized to be possible solely in the up conformation.3,4
Efficient Neutralizing Activity is Demonstrated by VHH Antibodies
There are numerous RBD-specific human monoclonal antibodies derived from former patients that have been isolated and reported to have uses in combatting coronaviruses. Strong neutralizing activities have been shown in animal models, as they inhibit the virus’ ability to infect cells by binding with the coronavirus spike protein.
This method is utilized to strengthen and interact with the host’s immune systems, contributing to increasing its efficacy in fighting the coronavirus. It has been proposed that only 1% may have high enough levels of antibodies naturally to neutralize the virus without needing intervention.5
These conventional antibodies have poor pharmacokinetics, are relatively large-sized (~160 kDa), are difficult to transport and store, and incur high production costs.3,4
Taking these challenges into account, researchers are investigating smaller biologicals like heavy-chain variable (VHH) antibodies, or nanobodies*, as alternatives to canonical antibodies due to their ability to bind to epitopes that are inaccessible to conventional antibodies and their higher stability.6
NbMS10 was a novel VHH antibody developed by Zhao et al. (2018) for the therapeutic treatment of MERS-CoV. NbMS10 bound to the S protein’s RBD with a high binding affinity (Kd, 8.71 x 10-10 M), blocking MERS-CoV from binding to a DPP4 mouse host-cell receptor.
A relatively low dose (ND50, 3.52 μg/mL) was discovered to be enough to neutralize the virus, hinting that NbMS10 could be a potential MERS-CoV therapeutic.4
However, applications for VHH antibodies may be limited because of their small size, which normally leads to a fast-renal clearance. In mice, NbMS10 was found to be cleared from the serum quickly, and, 10 days after the injection, the binding affinity for MERS-CoV RBD was completely lost.
NbMS10-Fc, a human-Fc-fused version, was designed by enlarging the VHH antibody from 16 kDa to 50 kDa in an attempt to overcome this problem.
Comparable binding affinity (8.71 x 10-10 and 3.46 x 10-10 M, respectively) and neutralizing activity (3.52 and 2.33 μg/mL, respectively) was shown by NbMS10-Fc to NbMS10, but the clearance rate was much slower, and stable binding for MERS-CoV RBD was still noted 10 days after injection.4
The data was comparable to that observed for RBD-specific conventional immunoglobulin Gs (IgG) (Kd, 7.12 x 10-10–4.47 x 10-10 M; ND50, μg/mL–ng/mL). Cross-neutralizing activity was also found to be high for both VHH antibodies compared to divergent MERS-CoV strains, with the ND50 ranging from 0.003–0.979 μg/mL and 0.003–0.067 μg/mL for NbMS10 and NbMS10-Fc, respectively.
This research suggests that VHH antibodies are potential broad-spectrum antivirals that could be beneficial in the ongoing SARS-CoV-2 pandemic.4
Wrapp et al. more recently isolated the VHH antibody SARS VHH-72 from a llama that was immunized with prefusion-stabilized coronavirus spikes targeted against SARS-CoV-1 RBD. SARS VHH-72 displayed nanomolar binding affinity and was able to neutralize pseudotyped SARS-CoV-1 viruses in vitro. These results were promising, so the researchers investigated how the VHH antibody affected SARS-CoV-2.
SARS VHH-72 was indicated to have a high affinity to SARS-CoV-2 RBD by surface plasmon resonance analysis. However, ELISA showed no interaction and the VHH antibody was unable to neutralize SARS-CoV-2 pseudoviruses.
Using a like approach to Zhao et al., the group engineered SARS VHH-72 into a bivalent Fc-fusion, SARS-VHH-72-Fc. A high binding affinity to SARS-CoV-2 RBD was shown by this modified VHH antibody, and it was able to neutralize SARS-CoV-2-(S) spike glycoprotein pseudoviruses. This provided more evidence that VHH antibodies are promising therapeutics for combatting the ongoing SARS-CoV-2 pandemic.1
Benefits of VHH Antibodies
VHH antibodies are the isolated VHH domains of heavy-chain only IgG2 and IgG3 antibodies produced by camelid species, such as alpaca, llama, and camel. These proteins consist of framework regions and three highly variable loops; H1, H2 and H3.
Researchers have shown that the H3 loop facilitates a wide range of bind specificities in VHH antibodies. This is due to the H3 loop normally being 3 or 4 residues longer than conventional antibodies, enabling the use of these protrusions in extending into “hidden” epitope cavities.
There is also more variation in amino acid sequences within the H3 loop for VHH antibodies when compared to conventional antibodies, in turn resulting in ~7% higher sequence diversity per residue.6
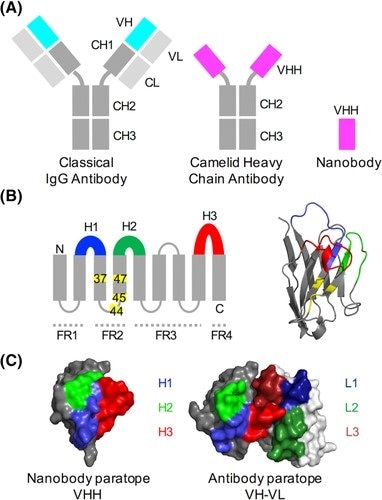
Figure 2. Structural features of conventional and camelid heavy‐chain antibodies (Ab). A, In an Ab, the antigen binds to the VH‐VL interface, while in the camelid heavy‐chain antibody the VH‐homologous VHH domain binds the antigen. In Abs, the VH and VL domains bind to each other and can only be produced in bacterial expression systems when joined by a peptide linker. B, Like the Ab VH domain, the secondary structure of the VHH domain consists of 9 beta-sheets separated by loop regions, 3 of which are hypervariable (shown in blue, green and red). Four framework regions (FRs) separate the variable loops; these are less sequence‐variable. Four positions known as the VHH‐tetrad are numbered and highlighted in yellow. Right: VHH domain with VHH‐tetrad positions in yellow. C, The antigen‐binding surface in VHH domains and Ab VH‐VL domains (aligned orientations). Image and figure legend courtesy of Mitchell & Cowell (2018)6 doi.org/10.1002/prot.25497 CC BY 4.0
As well as being structurally different, there are many other characteristics that make VHH antibodies more advantageous than canonical antibodies:3–7
Table 1.
| . |
. |
| Variable functionality |
VHH domain acts as a framework for recombinant antibody architecture, allowing the engineering of different structural formats. Tags can be added for purification or functional groups can be attached for the conjugation of drugs or fluorescent probes. |
| Small size (12–15 kDa) |
Less susceptible to steric hindrance due to their smaller size, conferring improved access to epitopes. VHH antibodies have good tissue permeability and can cross the blood-brain barrier, making them attractive drug delivery options. |
| Low production cost |
VHH antibodies can be produced recombinantly at high yields using bacterial, yeast, or mammalian expression systems. |
| High stability |
VHH antibodies have good solubility, high thermal and chemical stability, and higher resistance to proteases than conventional antibodies. High stability also allows nebulization and administration via an inhaler to directly reach the site of infection. |
Production of High-Affinity VHH Antibodies by Jackson ImmunoResearch
For the engineering of VHH antibodies, production optimization, purification and isolation are vital for the maximization of yield and the generation of high-quality, high affinity products that can be used for the treatment of patients.
Jackson ImmunoResearch Laboratories, Inc. is a specialist in the production of highly specific secondary antibodies and immunoreagents. It is dedicated to supporting manufacturers that generate VHH antibodies for therapeutics or diagnostics to treat novel viral infections, such as SARS-CoV-2.
A range of products has been developed by Jackson ImmunoResearch to enable the production of VHH antibodies and to optimize each stage of their development through practices such as screening VHH antibody candidates and tracking seroconversion.
Alpaca, camel and llama proteins can be detected with its Anti-Alpaca antibodies, and they are available with specificity for IgG (H+L), IgG, subclasses 2+3, or VHH domains.
There is use for them in several techniques such as flow cytometry, ELISA, immunofluorescence, and Western blotting. The design of the products aims to guarantee the production of high-quality candidates by optimizing VHH antibody.7,8
References and Further Reading
- Wrapp D., et al. (2020). Structural Bases for Potent Neutralization of Betacoronaviruses by Single-Domain Camelid Antibodies. Cell. https://doi.org/10.1016/j.cell.2020.04.031.
- www.bbc.com. (2020). Coronavirus Pandemic: Tracking the Global Outbreak. https://www.bbc.co.uk/news/world-51235105?utm_source=dlvr.it&utm_medium=twitter
- Mahase E. (2020). Covid-19: What Treatments are Being Investigated? British Medical Journal. https://doi:10.1136/bmj.m1252.
- Zhao G., et al. (2018). A Novel Nanobody Targeting Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Receptor-Binding Domain Has Potent Cross-Neutralizing Activity and Protective Efficacy Against MERS-COV. Journal of Virology. https://doi.org/10.1128/JVI.00837-18.
- https://www.nih.gov/news-events/nih-research-matters/potent-antibodies-found-people-recovered-covid-19
- Mitchell L. S., et al. (2017). Comparative Analysis of Nanobody Sequence and Structure Data. Proteins. https://doi.org/10.1002/prot.25497.
- www.jacksonimmuno.com. (2020). Camelid Immunology. https://www.jacksonimmuno.com/technical/products/groups/camelid
- www.jacksonimmuno.com. (2020). Introducing Anti-Alpaca IgG, VHH Domain Secondary Antibodies. https://www.jacksonimmuno.com/secondary-antibody-resource/wp-content/uploads/Anti-VHH-handout-US-online.pdf
- Zhang Z., et al (2020). Human neutralizing antibodies elicited by SARS-CoV-2 infection. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature PubMed: 32454513 DOI:10.1038/s41586-020-2380-z
* Nanobodies is a trademark of Ablynx N.V.
Acknowledgments
Produced from materials originally authored by Miranda Lewis from Jackson ImmunoResearch.
About Jackson ImmunoResearch Laboratories, Inc. 
Jackson ImmunoResearch Laboratories, Inc. specializes in the production and conjugation of affinity-purified secondary antibodies and purified immunoglobulins. Our products are sold primarily to scientists in universities and research institutes throughout the world who are conducting research in the plant, animal, and biomedical sciences.
The company is located in rural Pennsylvania, near the Amish country, about 40 miles west of Philadelphia. It was started in 1982 by scientists whose education and previous experience included research in cell biology, protein chemistry, microbiology, and product development in immunology. Our goal is to provide other scientists with the newest, largest selection of, and highest quality of secondary reagents, with the best technical and customer services possible. The products are for research use only, and not for diagnostics or therapeutics. They are not primary antibodies, nor are they medical devices.
We hope this site will provide not only information about our company and our products, but also answer some technical questions you may have regarding the use of our reagents.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.