Sponsored Content by GenScriptReviewed by Maria OsipovaSep 8 2022
A novel immunotherapy tool has emerged in the last ten years. T cells engineered to express chimeric antigen receptors, commonly known as CAR T cells, have demonstrated the ability to help patients suffering from blood cancer.
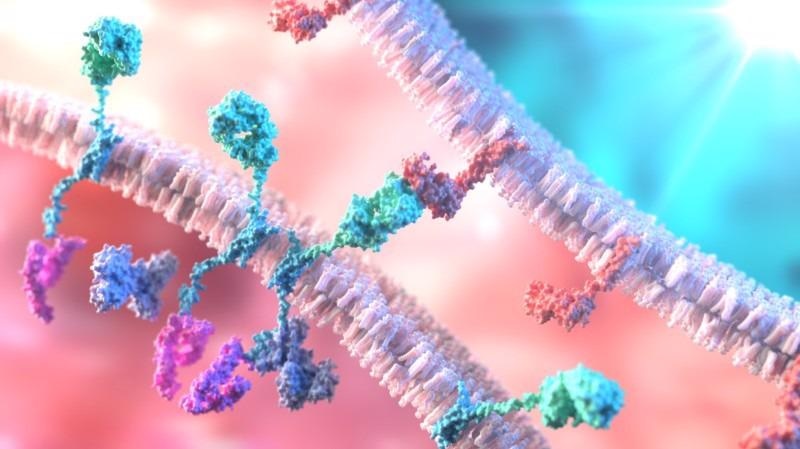
T cells engineered to express chimeric antigen receptors (CAR-T cells; bottom left) can bind to tumor cells (top right) and attack blood cancers. Engineering CAR-T cells using CRISPR is now possible using a new technique and high-quality ssDNA. Image Credit: Shutterstock.
A groundbreaking study1 in 2011 utilized second-generation CAR T cells to accomplish sustained T cell activation and remission for most of the patients tested.
The following year came another significant breakthrough, as two groups described2,3 a novel gene-editing tool known as CRISPR-Cas9 exhibited its use in eukaryotic cells. These two reports kick-started a new era in gene editing.
While gene editing had the potential to help progress cell engineering technology, it would be a number of years before CAR T cells and CRISPR were to cross paths.
Finding the path
Early on, CAR T cells demonstrated exceptional cancer-killing potential, but producing these cells was a clumsy and complicated process.
Initially, T cell engineering was contingent on lentiviral or retroviral vectors to help deliver DNA fragments into a cell for homologous recombination. Viral vectors facilitate stable integration of DNA fragments and prolonged expression.
However, the clinical-grade reagents required for such vectors can be expensive, and the vectors can only transport a limited amount of DNA which has the potential to integrate into the genome at random.
To help advance CAR T cell therapy, researchers had to find a more effective way to engineer long CAR sequences.
CRISPR drives the CAR
From the start, CRISPR promised to be the perfect solution to engineering a T cell. It is a relatively straightforward process, with minimal off-target effects, and works across an extensive range of cell types. But there is one area where CRISPR has been held back.
CRISPR-Cas9 is effective at producing gene knockout mutations by generating targeted double-stranded DNA (dsDNA) breaks that the cell’s non-homologous end-joining pathway incorrectly repairs.
However, CRISPR editing can be extremely inefficient when it comes to knocking-in an exogenous DNA sequence using homology-directed repair mechanisms. Yet, inserting DNA is vital to effectively engineering CAR T cells.
“Easi-CRISPR is a much better tool when it comes to homology directed repair,” says Channabasavaiah Gurumurthy, a gene engineer at the University of Nebraska Medical Center in Omaha, who co-invented Easi-CRISPR in 2017.
Gurumurthy and his team found that, when it comes to inserting DNA into a cell by applying CRISPR, long single-stranded DNA (ssDNA) is a more efficient template than double-stranded.
With Easi-CRISPR, long ssDNA is inserted along with a preassembled complex comprised of Cas9 and guide RNAs, generating higher rates of on-target editing and reduced rates of off-target editing.4
In conjunction with previous reports demonstrating chemically synthesized single guide RNAs (sgRNA) with 2’-O-methyl and phosphorothioate end modifications improved intracellular stability and editing efficiency in primary cells5, suddenly it appeared that CRISPR-Cas9 could be a suitable tool for the effective production of both deletions and insertions within T cells.
All this hard work came together last year when Alexander Marson, Gurumurthy, and colleagues utilized Easi-CRISPR to reprogram the structure and function of human T cells without the necessity for viral vectors6.
This study showed that CRISPR editing of T cells with the use of ssDNA as a homology-directed repair template was more precise and efficient for large gene insertion, with less off-target integration than dsDNA templates.
Looking to the future
While this breakthrough was exciting, there was one key consideration.
“Long ssDNA sequences are difficult to produce in the lab, especially at the high concentrations necessary for gene editing experiments,” says Theodore Roth, a member of Marson’s lab and lead author of the study6.
Numerous companies and academic developers are now attempting to solve this problem, effectively creating methods to generate large amounts of long ssDNA.
Recently, GenScript, a biotechnology company based in Piscataway, NJ, known for its world-leading DNA synthesis technologies, has started to offer ssDNAs several thousand nucleotides long, in quantities up to 100 micrograms — perfectly suited for T cell reprogramming utilizing CRISPR technology.
GenScript is also one of the few companies that offers total CRISPR solutions, including HPLC-purified, chemically synthesized sgRNAs with end modifications to improve CRISPR editing.
Gene editing is revolutionizing the way researchers approach cell engineering. Methods such as Easi-CRISPR, along with advancements in DNA and RNA synthesis, will further enhance CAR T cell engineering efforts, ultimately enhancing cancer therapy and long-term human health.
References
- Porter, D.L., et al. N Engl J Med 365, 725–733 (2011)
- Jinek, M., et al. Science 3337, 816–812 (2012).
- Cong, L., et al. Science 339, 819–823 (2013).
- Miura, H., et al. Nat Protoc. 13, 195–215 (2018).
- Hendel, A. et al. Nat. Biotechnol. 33, 985-989 (2015).
- Roth, T.L., et al. Nature 559, 405–409 (2018).
About GenScript
Genscript is the world’s leading biotech company providing life sciences services and products. With gene synthesis, peptide, protein, antibody and preclinical drug development service capabilities, we are internationally recognized as a leading biotech company specializing in fundamental life sciences research and early-phase drug discovery services. As of 2018, more than 30,000 peer-reviewed journal articles cited GenScript’s services and products, making GenScript the most frequently cited biotech company in the world.
After almost two decades of fast growth in developing biological reagents, the company has expanded its business into immunotherapy, CDMO, laboratory equipment, and microbial industry to further fulfill its mission in making people and nature healthier through biotechnology.
Founded in 2002 in New Jersey, United States, GenScript serves as a partner for researchers in basic life sciences, translational and biomedical fields as well as early-stage drug development.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.