Sponsored Content by GenScriptReviewed by Maria OsipovaNov 16 2022
The scientific symposium “Therapeutic Applications of Non-Viral RNA: Therapy Strategies” at this year’s ASGCT annual meeting involved researchers from the University of Pennsylvania and Moderna Therapeutics who gave detailed insight into the benefits of RNA technology for drug development.
Advantages of mRNA
First, Hamideh Parhiz, PharmD, Ph.D., of the University of Pennsylvania, delivered a talk entitled “Targeted Lipid Nanoparticle Platform for in vivo Delivery of mRNA Therapeutics.” Dr. Parhiz began by addressing the primary advantages of mRNA for therapeutic techniques over other macromolecules like DNA and proteins. From dose limitations to the rate of expression and ease of delivery, mRNA molecules exceed other macromolecules as a therapeutic approach.
Unlike DNA, which must pass through the cell membrane and cytoplasm to enter the nucleus, mRNA molecules are immediately processed by the cytosolic translation machinery, meaning that they require fewer molecules and accelerate protein production.
Furthermore, genomic integration, which can be considered a danger with some DNA therapies, is completely prevented, resulting in transient protein production that can be more readily regulated to produce desirable therapeutic outcomes.
Dr. Parhiz stated that the adaptability of mRNA, which allows for the rapid creation of either secreted, intracellular, or membrane-bound proteins, is a significant advantage of this therapeutic vehicle.
Finally, advancements in mRNA synthesis procedures, like the use of chemically modified nucleosides (e.g., pseudouridine), in conjunction with better purification methodologies, have allowed for the practical use of in vitro transcribed (IVT) mRNA macromolecules as drugs.
Collectively, these beneficial features and synthetic breakthroughs have considerably accelerated the therapeutic development workflow and, therefore, the delivery of mRNA therapies to the clinic by frequently utilizing lipid nanoparticles (LNPs) as a favored delivery vehicle.
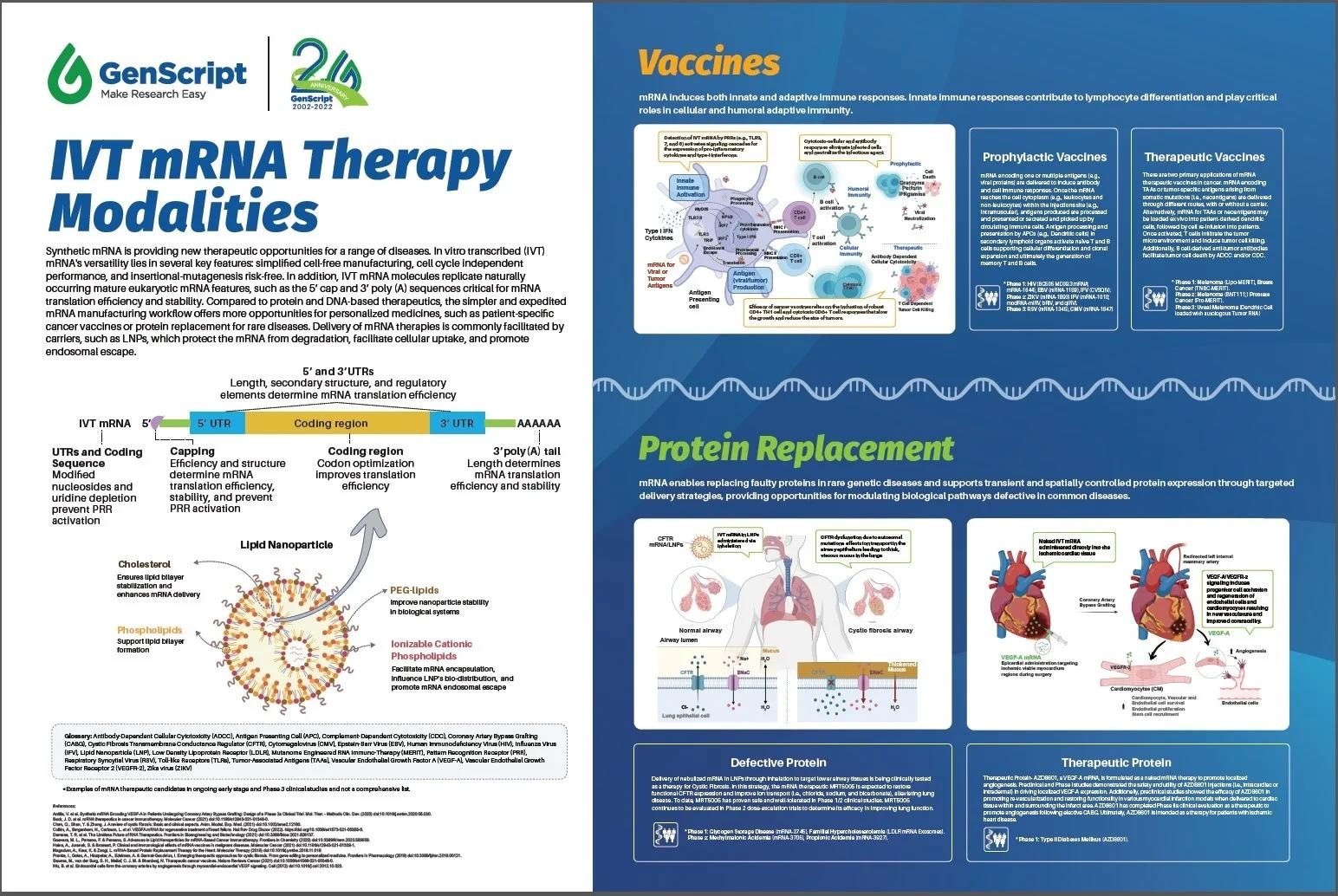
IVT mRNA Therapy Modalities poster. Overview of IVT mRNA structural and delivery advances enabling today’s therapies and mRNA therapy modalities advancing through clinical trials. Image Credit: GenScript
In addition to their critical role in safeguarding mRNAs from degradation, LNPs can be altered to allow therapeutically relevant tissues to be targeted.
According to Dr. Parhiz, whose research focuses on optimizing LNP tissue targeting, one major downside of this delivery vehicle is its preference for liver absorption. Dr. Parhiz has enabled the targeted delivery of mRNA to diverse cell types (e.g., endothelial and T cells) by inventing a tailored LNP-mRNA platform that leverages antibody specificity.
In recent times, in a preclinical model of cardiac fibrosis, this approach enabled the in vivo targeting of T cells to generate temporary CAR-T cells with specificity towards activated fibroblasts (anti-Fibroblast Activated Protein or FAPCAR) (Rurik et al. 2022).
Dr. Parhiz agreed that the in vivo synthesis of CAR-T cells using mRNA-targeted LNPs is a powerful strategy that would help patients for whom traditional in vitro CAR-T cell production is impractical because of low T cell levels.
Another interesting treatment area where IVT mRNA medicines can have a substantial impact is monogenic disorders. “Strategies for Developing mRNA-Based Therapeutics for Rare Diseases” was the topic of a presentation by Dr. Lisa Rice, a scientist on Moderna Therapeutics’ strategy team.
Moderna
A variety of IVT mRNA synthesis advancements have been introduced at Moderna to enable the effective manufacturing of mRNA therapeutics like the now well-known COVID-19 vaccine, mRNA-1273, or Spikevax.
In addition to enabling the development of preventative vaccines, Moderna’s platform also aids in the creation of mRNA treatments for a variety of other purposes, including the creation of cancer vaccines, intratumoral immune-oncology, localized regenerative therapies, and more.
Dr. Rice discussed how numerous developments in mRNA chemistry, sequencing, targeting elements, transport schemes, and enhanced manufacturing techniques have boosted Moderna’s IVT mRNA pipeline.
Moderna is using LNPs to deliver mRNA therapies intravenously (i.v.) for rare diseases. Dr. Rice also explained how the IVT mRNA and protein product’s pharmacokinetics (i.e., half-life) must be modified and optimized for maximum results in protein replacement applications.
To this end, Moderna’s scientists closely fine-tune the translation initiation fidelity to ensure the generation of the proper and functional protein product, which is often enzymes in rare diseases. Moderna’s team also incorporates microRNA target regions into the IVT mRNA’s UTR sequences to accomplish tailored protein expression in cells and tissues.
Finally, the immunogenicity of IVT mRNA is regulated by chemical changes and manufacturing procedures. Moderna’s team is now undertaking preclinical investigations to develop IVT mRNA therapy for Phenylketonuria (PKU), a rare condition caused by hereditary abnormalities in the gene encoding phenylalanine hydroxylase.
Reference
- Rurik et al. CAR T cells produced in vivo to treat cardiac injury. Science (2022). DOI: 10.1126/science.abm0594.
About GenScript
Genscript is the world’s leading biotech company providing life sciences services and products. With gene synthesis, peptide, protein, antibody and preclinical drug development service capabilities, we are internationally recognized as a leading biotech company specializing in fundamental life sciences research and early-phase drug discovery services. As of 2018, more than 30,000 peer-reviewed journal articles cited GenScript’s services and products, making GenScript the most frequently cited biotech company in the world.
After almost two decades of fast growth in developing biological reagents, the company has expanded its business into immunotherapy, CDMO, laboratory equipment, and microbial industry to further fulfill its mission in making people and nature healthier through biotechnology.
Founded in 2002 in New Jersey, United States, GenScript serves as a partner for researchers in basic life sciences, translational and biomedical fields as well as early-stage drug development.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.