This article explores the work to activate the PEEK surface of medical devices, aiming to significantly enhance the PAD printing process to achieve complete print adhesion.
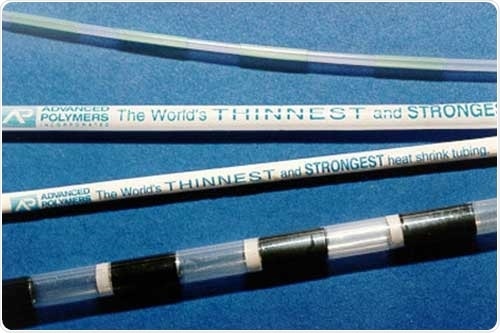
Image Credit: Henniker Plasma
Print ink adhesion (PAD)
The PAD Printing technique places a very thin layer of ink onto the surface, but this technique proved sub-optimal when used with medical devices manufactured from polyetheretherketone (PEEK), for example, catheters, microcatheters, nasogastric feeding tubes and endotracheal tubes.
Polyetheretherketone is an engineered thermoplastic that exhibits exceptional heat resistance. PEEK’s glass transition temperature is 144 °C, while its melting point is 335 °C. It is generally melt-processed at 370 °C.
While these properties provide a range of tangible benefits to a design engineer, they can also lead to a secondary assembly and decorating issues, particularly in terms of bonding, printing, coating and painting.
Henniker Plasma has been working to identify a viable solution to these issues.
The solution
One of the fundamental requirements for successful ink adhesion lies in appropriately spreading the ink onto the substrate. Spreading occurs if the ink’s surface tension is lower than the surface free energy of the part.
The team began by confirming there were no underlying contamination issues. This was done by determining the surface free energy of a number of PEEK devices via a range of in-house surface test methods. This is a frequently employed step in Henniker Plasma’s process development method.

Image Credit: Henniker Plasma
The importance of surface energy
Surface energy can be understood as the surplus energy at the surface of a material versus the bulk material itself.
Should the liquid’s molecules be attracted to each other more strongly than they are to the surface, the liquid will tend to form beads rather than wetting the surface. A larger attraction to the surface will cause the liquid to spread out more, however.
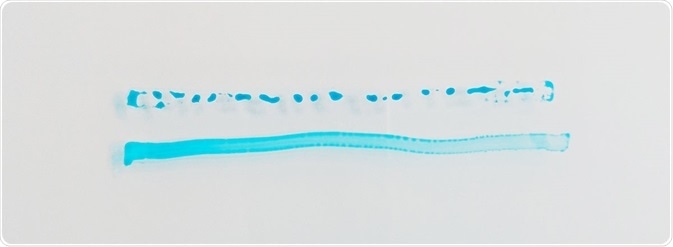
Image Credit: Henniker Plasma
A surface with higher surface energy will wet more easily. As the ability to wet a surface can be understood as a simple definition of the adhesion characteristics of the surface, it is, therefore, easier to glue, paint, print or bond to that particular surface.
Measurements revealed surface energy values between 30-35 dynes for untreated PEEK, while the most commonly utilized inks in this process exhibited surface tension values approximately 25% higher.
Plasma surface treatment
A number of plasma treatments were investigated to determine the most rapid and cost-effective means of enhancing the PEEK devices’ surface wettability.
Once initial trials had been completed, it was possible to put a simple and effective process in place to increase the PEEK surface energy to more than 72 dynes. This was possible with a total process cycle time of less than two minutes.
Ensuring satisfaction
Henniker Scientific’s robust combination of experience and expertise in the development and optimization of low surface energy materials - coupled with the company’s capacity to undertake rapid testing and develop a viable process - has proved invaluable to the teams they work with.
Once it had been developed and tested, the process was transferred to Henniker Plasma’s in-house contract treatment team, affording clients a rapid, cost-effective solution to their printing problems.
About Henniker Plasma
Henniker – Passionate about Plasma®
We are an experienced, dynamic and expanding company already established as a leading UK manufacturer of plasma treatment systems for cleaning, activation and coating.
Our success is built around an exceptional body of knowledge and expertise, backed by highly trained and dedicated staff who understand your application in considerable detail. Our standard configurations cover most applications but we understand too that no two samples or surfaces are the same. That’s why, uniquely, we offer a wide range of options that allow us to customise any standard system for your exact requirement.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.