SWATH DIA (data independent acquisition) has emerged as an LC-MS/MS workflow that capitalizes on the speed and sensitivity gains that have been achieved with modern mass spectrometers. SWATH DIA can provide identification of vast numbers of proteins, peptides and metabolites in a more reproducible manner than conventional data dependent acquisition (DDA) methods. With SWATH DIA, high quality, high resolution MS/MS data not only provide identification but are also the secret behind highly accurate and precise quantification.
Now, Zeno SWATH DIA marks a significant step-change in data independent acquisition delivering a high depth of coverage, particularly on low abundance species, quickly and robustly.
Exclusively on the ZenoTOF 7600 system from SCIEX, the Zeno trap technology has expanded the reach of assays for many applications through sensitivity gains provided through MS/MS duty cycle enhancements. With the Zeno trap, nearly >90% duty cycles are now achievable for all ions regardless of mass, charge, or LC flow rate. This provides sensitivity gains of 4- to 20-fold without compromising other important performance specifications such as resolution, mass accuracy, dynamic range and speed.
Zeno SWATH DIA exploits these gains, revealing up to thousands more identified and quantified analytes in a shorter period with superior precision.
Achieving deeper coverage, Zeno SWATH DIA provides the maximum information in the minimum time. The 6-10x sensitivity gains in MS/MS mode that the Zeno trap delivers by duty cycle improvements provide as much as 3x more identified proteins, and approximately 3-6x more quantified, at loads smaller than 20 ng.
This enables a more complete understanding of the underlying biological transformations. The maximum data is acquired from each precious sample with Zeno SWATH DIA.
SWATH DIA is now the preferred method for large-scale proteomics and other studies. The gains in sensitivity expand the application space to incorporate routine execution of these studies using microflow, or even high-flow, chromatography for more rapid and robust analyses.
Higher sensitivity leads to higher quality data for lower levels of analyte. Nanoflow chromatography Zeno SWATH DIA delivers reproducible quantitative and qualitative data at sample levels approaching single-cell analysis.
Regarding single-cell analysis, discrete cellular states and events can be distinguished from bulk proteomic discoveries, enabling an improved understanding of cellular heterogeneity.
Reproducible and accurate quantification at these levels is essential, but it is frequently more challenging or labor-intensive to attain these abundance measurements from labeling methods or profiling data.
Zeno SWATH DIA quantification utilizing MS/MS data bypasses numerous issues by delivering higher intensity spectra and high MS/MS scan rates with a broad dynamic range.
This article reviews the technology behind Zeno SWATH DIA and the crucial mass spectrometer performance attributes that enable this. Examples show trace levels of analytes with higher throughput and precision than previously seen.
Data-dependent acquisition (DDA)
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) is popular for the characterization of unknown compound mixtures. Data-dependent acquisition (DDA) is conventionally employed to acquire MS/MS data on as many compounds as possible.
Due to its untargeted sample analysis, DDA is well-established and employed in many applications. DDA facilitates quantification at the MS1 level utilizing label-free methods or at the MS/MS level using labeled methods, with the latter demanding additional sample preparation protocols and costs.
One key disadvantage of DDA is that datasets can be incomplete due to the selection of only precursor ions that match specific criteria for MS/MS analysis. Detection reproducibility can also be affected.
Small changes in retention times between runs can impact the population of precursor ions that enter the instrument during each cycle, which also affects the subset of compounds analyzed. However, while the matrices remain complex, the capability for ionization and detection has improved.
The instrument does not have the time to sample each potential precursor that enters the system. A target list of precursors may be utilized to counteract this, but this removes the advantage of a truly unbiased and global approach.
Missing gaps and peaks are frequently observed when comparing multiple DDA data sets. This can be especially damaging for lower-level analytes and low replicate numbers.
Image Credit: SCIEX
Data-independent acquisition (DIA)
The data-independent acquisition (DIA) strategy named SWATH DIA was published for the first time in 2012.1 SWATH DIA involves all ionizable precursors being analyzed by MS/MS, irrespective of abundance or other criteria.
This leads to greater repeatability from a complete data set that contains fragment data for all precursor ions.
SWATH DIA was initially utilized for proteomics experiments, but the workflow has been implemented for various additional applications, such as environmental screening, metabolomics, forensics, food testing, and pharmaceutical analysis.
SWATH DIA uses broader precursor selection windows for MS/MS that enable several compounds to go through simultaneously. These windows are stepped across the whole precursor mass range, meaning all precursor masses are fragmented for every cycle.
MS/MS spectra produced through SWATH DIA are usually more complex than DDA's. SWATH DIA utilizes many windows to reduce the number of precursors within each window to enhance specificity.
Fast MS/MS scanning enables iteration through all windows within each cycle while continuing to cover a broad mass range. The utilization of variable window widths further enhances the selectivity of SWATH DIA.
With variable windows, the width of each precursor window is altered, corresponding to the complexity of data acquired within that mass range. More expansive windows are utilized when analytes are sparsely populated, and very narrow windows are used when analyte density is greatest.
This improves the percentage of high-quality identified and quantified precursors. The capability of SWATH DIA was proven in a landmark multi-laboratory proteomics study in 2017.
In this study, 11 laboratories from various locations across the globe showed that SWATH DIA could be employed for consistently detecting and quantifying proteins for the sensitive, reproducible, and large-scale identification and quantification of complex proteomics samples.2

Image Credit: SCIEX
Zeno trap enabling Zeno SWATH DIA
Zeno SWATH DIA combines the Zeno trap's power with SWATH DIA.3,4 Like SWATH DIA, Zeno SWATH DIA uses MS/MS data to identify and quantify analytes. As a result, the fast acquisition of high-quality MS/MS data across the whole precursor ion space is essential for operation.
In Zeno SWATH DIA, when the Zeno trap is activated, it boosts the MS/MS sensitivity for each acquired variable window.5 The Zeno trap provides a 4-20x sensitivity gain for Zeno SWATH DIA while preserving other vital performance attributes.6
Fast scan rate
Zeno SWATH DIA utilizes fast scanning and detection to maximize the total number of high-quality MS/MS spectra produced per cycle. This allows a more significant number of variable windows, increasing specificity and confidence in the total number of analytes identified and quantified.
Faster scanning also allows quicker LC run times, vastly enhancing throughput and laboratory productivity.
Resolution and mass accuracy
Co-eluting isobaric analytes, contaminants, and high background can affect the quantification of analytes, particularly at the MS level, even when extremely high-resolution instruments are utilized. Zeno SWATH DIA maximizes the precision and accuracy of quantification by using the selectivity of MS/MS.
When used with the highest scan rates, these attributes (mass resolution and accuracy) are maintained to preserve the maximum number of analytes identified and quantified.
Dynamic range
Both inter-scan and intra-scan dynamic ranges are crucial for identification and quantification. Zeno SWATH DIA incorporates a wide intra-scan dynamic range that detects low-level analytes in the presence of high-abundance analytes within the same scan, with no peak distortion or saturation.
Zeno SWATH DIA also has a wide inter-scan dynamic range (linear dynamic range, or LDR) which allows the detection and quantification of analytes that span a range of abundances within one run.
Zeno SWATH DIA
The 4-20x increase in MS/MS sensitivity delivered by the Zeno trap, while all other necessary performance specifications are maintained, leads to more high-quality MS/MS spectra. With Zeno SWATH DIA, this translates from the raw MS/MS to the MS/MS XICs (extracted ion chromatograms) and the total peptide ion current.
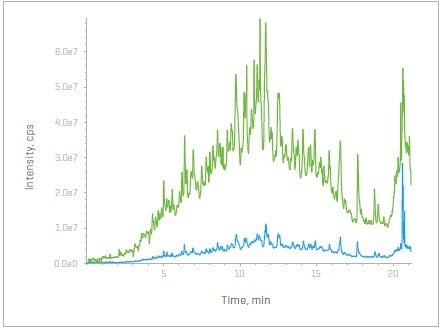
Figure 1. Total ion chromatograms (TICs) with and without the Zeno trap activated – SWATH DIA (bottom, blue) and Zeno SWATH DIA (top, green). Image Credit: SCIEX
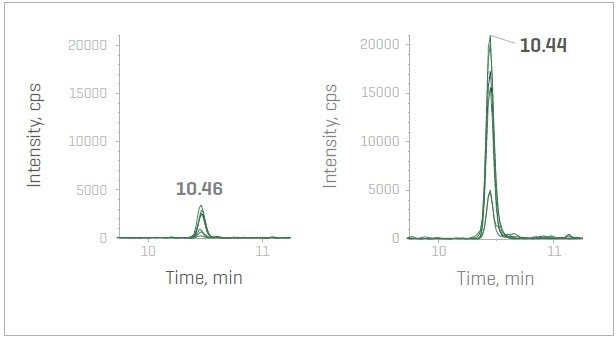
Figure 2. MS/MS XICs with and without the Zeno trap activated for peptide ITVTSEVPFSK (P35268) – SWATH DIA (left) and Zeno SWATH DIA (right). Image Credit: SCIEX
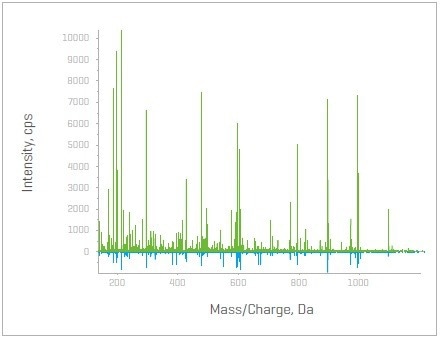
Figure 3. MS/MS spectra with and without the Zeno trap activated for peptide ITVTSEVPFSK (P35268) – SWATH DIA mirror (bottom) and Zeno SWATH DIA (top). Image Credit: SCIEX
More proteins identified and quantified
The 4 -10x increase in MS/MS sensitivity for peptides provided by the Zeno trap, while all other critical performance specifications are maintained, leads to more high-quality MS/MS spectra. An even greater number of peptides and proteins can be identified and quantified with higher precision with Zeno SWATH DIA.7
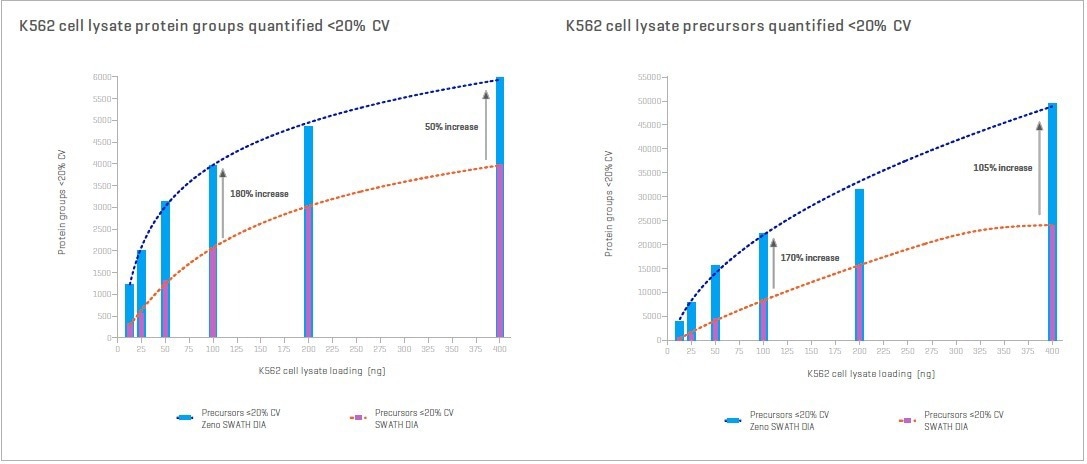
Figure 4. Protein and peptide precursor loading curves for 45 min gradient with and without Zeno MS/MS. Proteins and peptides quantified increase as sample loading rises. Image Credit: SCIEX
Library-free for protein ID
DDA utilizes a database search method in which MS/MS fragment ions and precursor ions are algorithmically searched against FASTA files. However, traditional SWATH DIA utilizes an MS/MS library for matching MS/MS data for protein and peptide identification.
This library is experimentally produced in advance from numerous DDA experiments. With recently developed algorithms, users can go “library free”, by employing an in-silico generated library. This removes the preliminary experiments needed to create the spectral library.
Using Zeno SWATH DIA data with the library-free method in DIA-NN8 allows a quicker and more streamlined protein identification workflow with excellent coverage depth.9
The library-free Zeno SWATH DIA approach identifies and quantifies many proteins and peptides compared to searching an experimentally generated library.
Utilizing microflow chromatography, this was benchmarked against the experimental library approach employing two different large libraries with very similar observed performance. Approximately 93% of the IDs are identical when assessing the overlap in protein IDs.
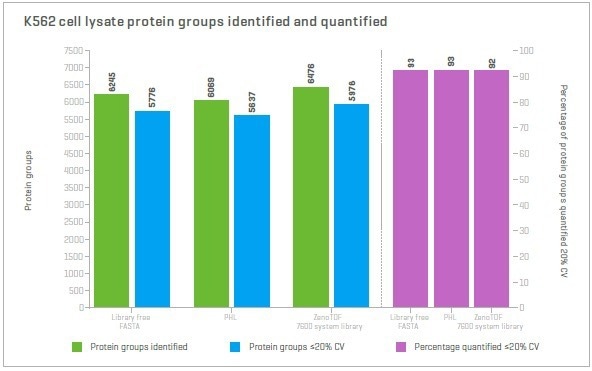
Figure 5. Comparing protein identifications using different approaches. Zeno SWATH DIA data were processed using two experimentally created libraries and a library-free approach. The number of proteins identified and quantified at ≤20% CV are very similar for all three approaches. Image Credit: SCIEX
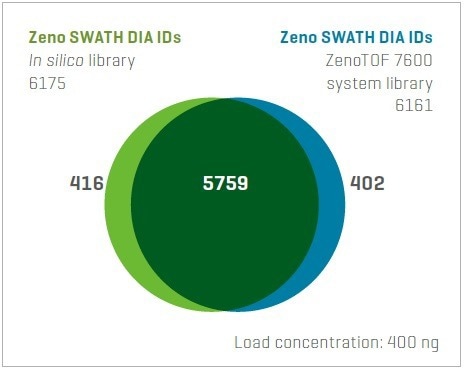
Figure 6. Similarity in protein identifications from Zeno SWATH DIA using an experimentally generated library and the library-free approach (in silico library). The overlap in protein identifications is very high, with nearly the same overall number identified by each. Image Credit: SCIEX
Nanoflow Zeno SWATH DIA for ultimate sensitivity and coverage
Nanoflow chromatography is often utilized when scientists seek maximum sensitivity from their LC-MS/MS experiments. With Zeno SWATH DIA, the improvements provided by the Zeno trap raise these sensitivity gains to new heights.
Using Zeno SWATH DIA and the extremely high sensitivity of nanoflow chromatography allows the identification and quantification of thousands of proteins. At loadings of 12.5 ng of K562 cell lysate, more than 5,000 proteins can be identified, with 80% having a CV of less than 20%.
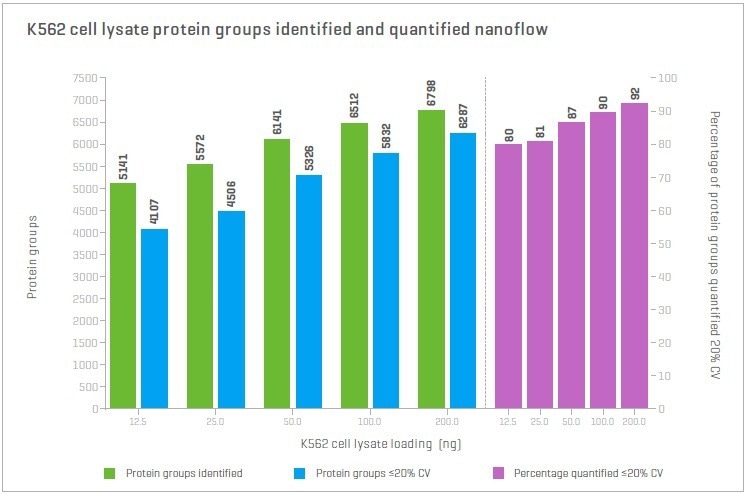
Figure 7. Proteins identified and quantified at different loadings of K562 digest for a 1-hour gradient. The number of proteins identified at <1% FDR and quantified at ≤20% CV using Zeno SWATH DIA across a range of loadings was studied. At all loads, >80% of proteins identified were also quantified. Image Credit: SCIEX
Nanoflow Zeno SWATH DIA sensitivity – towards single cell analysis
Proteomic results from cellular extracts represent all cellular states within the extract, but individual cells are dynamic biological entities frequently demonstrating vast variability in their molecular activities. As a result, there has been an increasing push towards single-cell analysis.
There is a need to gain insight into the different stages of individual cell growth and health, in addition to discrete cell-to-cell interactions. With Zeno SWATH DIA, ultra-low-level analyses make single-cell analysis feasible for numerous cell types.
Zeno SWATH DIA identifies approximately 1,000 proteins and quantifies around 400-500 proteins for protein loads of only 250-500 pg of the cell line. Compared to SWATH DIA, Zeno SWATH DIA identifies approximately 3x more proteins and quantifies as much as ~5x more for a 500 pg K562 cell lysate protein load.
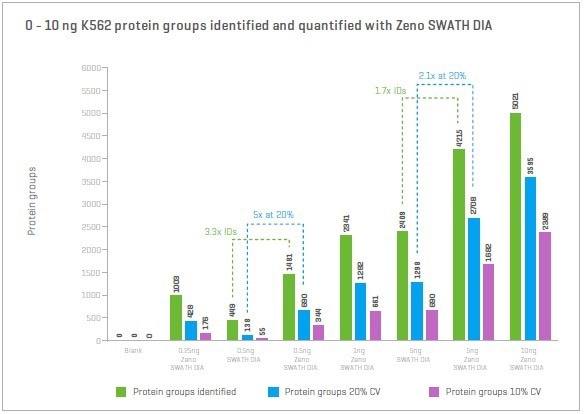
Figure 8. Proteins and peptides were identified and quantified with Zeno SWATH DIA using nanoflow chromatography and low sample loadings. Even at loading a single cell (250-500 pg), Zeno SWATH DIA identifies and quantifies hundreds of proteins. Image Credit: SCIEX
Nanoflow Zeno SWATH DIA reproducibility – towards single-cell analysis
Run-to-run variabilities in identifying and quantifying proteins can become more extreme as sample loading decrease. Due to the data-independent nature of Zeno SWATH DIA, run-to-run repeatability and reproducibility are maximized.
Zeno SWATH DIA demonstrates excellent sensitivity and reproducibility over multiple days, even for low sample loadings, with < 8% inter-day RSD for 500 and 1000 pg loadings. At sample loads of only 250 pg, approximately 1000 proteins are reproducibly identified.
Inter-day precision of quantification for all proteins is < 20% CV. For a low abundant peptide, it is quantified with < 1% CV, as demonstrated in the inset.
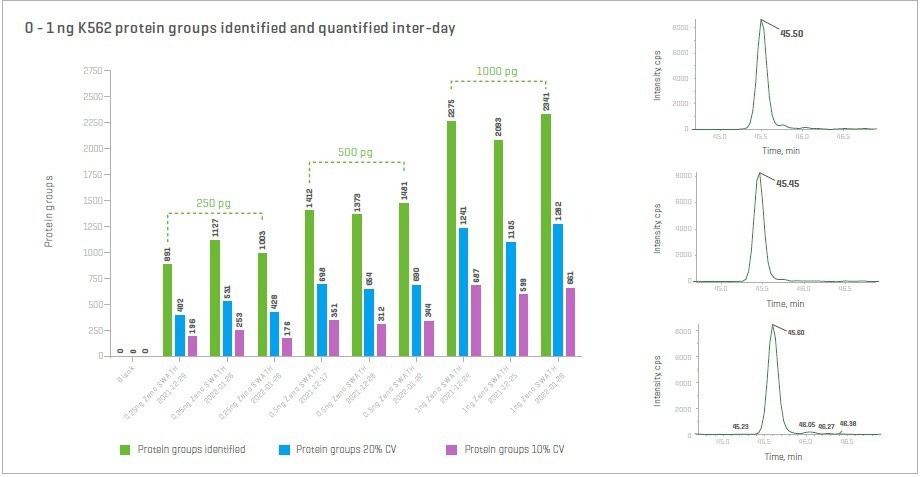
Figure 9. Proteins were identified and quantified with Zeno SWATH DIA using nanoflow chromatography across multiple days. High precision and reproducibility are observed for multiple injections of the same sample over multiple days, even at very low sample loads. Inset: MS/MS fragment ion signals for 3 different injections, spanning multiple days, for a 250 pg sample load. A %CV of only 0.73% is observed for summed fragment ions. Image Credit: SCIEX
Higher flow rates, higher productivity
Due to its quantitative reproducibility and depth of proteome coverage, SWATH DIA has rapidly become the chosen method for large-scale proteomics studies. Zeno SWATH DIA takes this further with the additional sensitivity gains the Zeno trap provides.
These sensitivity gains can enhance throughput and robustness by converting chromatography to higher flow rates.7
Zeno SWATH DIA drives throughput, and with rapid microflow gradients, it delivers 3000-6000 quantified proteins, dependent on the selected gradient and sample load.
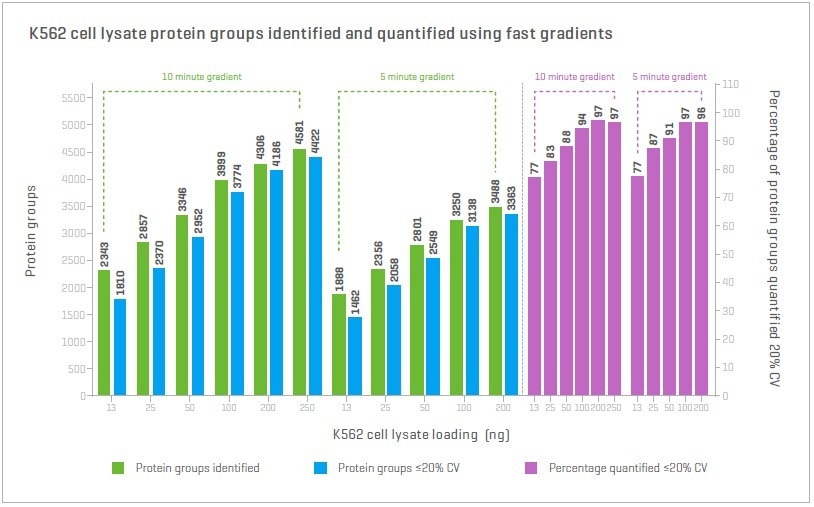
Figure 10. Comparing 10 min and 5 min gradients. The number of proteins identified at <1% FDR and quantified at <20% CV across a range of loadings was studied. These fast gradients also quantify 80-90% of proteins identified. Image Credit: SCIEX
Faster analysis with lower sample amounts
To deliver the same level of sensitivity as nanoflow experiments, high-flow LC-MS/MS experiments utilizing robust, analytical flow rate chromatography usually demand higher sample loadings. This sample requirement often limits the scope and size of any large-scale proteomics study utilizing high flow rates.
The increased sensitivity of Zeno SWATH DIA enables rapid and sensitive large-scale proteomics studies utilizing higher flow rates and lower sample loadings.
At high flow rates utilizing fast, five-minute gradients, it has been demonstrated that Zeno SWATH DIA delivers the same results using an average of only 1/10 the amount of sample injected, compared to SWATH DIA.
Zeno SWATH DIA also substantially raises the number of proteins identified and quantified at high flow when injecting identical amounts, providing hundreds and thousands more identified and quantified proteins.5
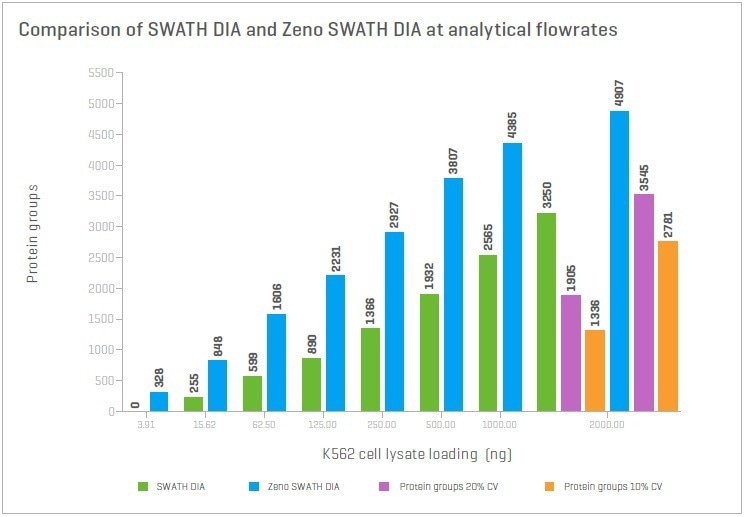
Figure 11. Comparison of SWATH DIA with Zeno SWATH DIA for high flow rate proteomics experiments (K562 using 800 μL/min, 5 min gradient). Left: Zeno SWATH DIA identifies and quantifies significantly more proteins and peptides than SWATH DIA across many different sample loading amounts. Right: Comparison of average number of IDs across 3 replicates (faint gray), number of consistent IDs (dark gray), quant < 20% CV (medium gray), quant < 10% CV (light gray). Image Credit: SCIEX
High-throughput plasma proteomics
LC-MS/MS proteomics studies can discover critical biological insights, but it can be tough to collect deep proteome coverage at the scale required to increase the size of a cohort study.
Zeno SWATH DIA surpasses the performance of conventional, deep, untargeted, proteomics strategies in terms of precision, depth, and throughput.10,11
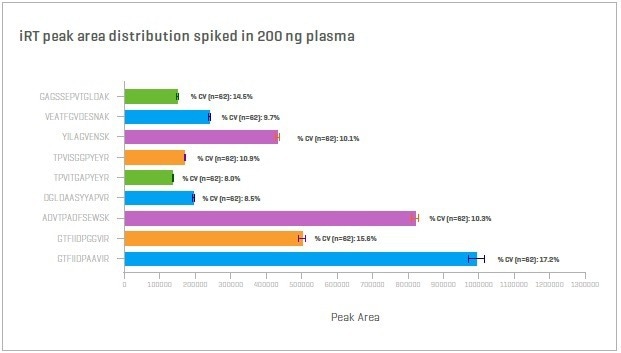
Figure 12. 200 ng non-depleted plasma spiked with iRT peptides. Run over two non-consecutive weeks to a total of 62 injections. iRT precision shows percentage CVs of less than 20%. Image Credit: SCIEX
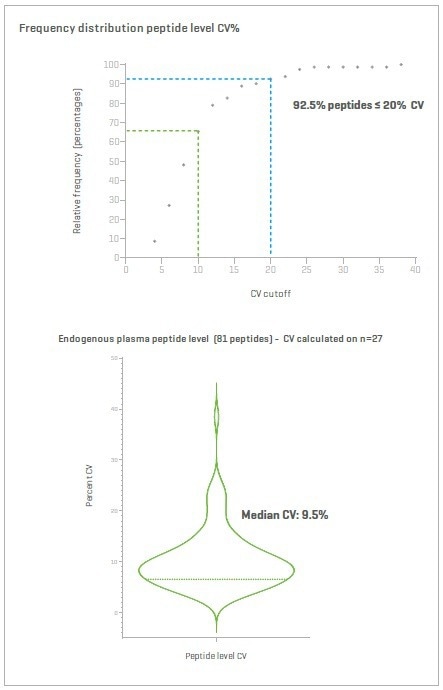
Figure 13. 200 ng non-depleted plasma spiked with SIL peptides for absolute quantification. The 81 endogenous light peptides are shown with a measure of CV distribution, both absolute and median. Image Credit: SCIEX
Untargeted metabolomics
High-confidence compound ID is undoubtedly the most significant obstacle researchers must overcome in untargeted metabolomics. This is primarily because of the ever-growing metabolome that is still to be annotated in spectral libraries, as well as the dynamic range of metabolites in a biological sample.
The depth of metabolomics coverage was restricted by sample size, MS/MS spectral richness, and sensitivity for quantifying low-abundance metabolites.
The ZenoTOF 7600 system has addressed all these challenges, which delivers fast and deep metabolite profiling with the Zeno Trap and Orthogonal fragmentation provided by EAD DDA.
Zeno SWATH DIA is a powerful approach to marry targeted and untargeted metabolomics through in-depth, comprehensive, and quantitative metabolome coverage. This saves significant resources and time for metabolomics researchers in pursuing biomarkers.
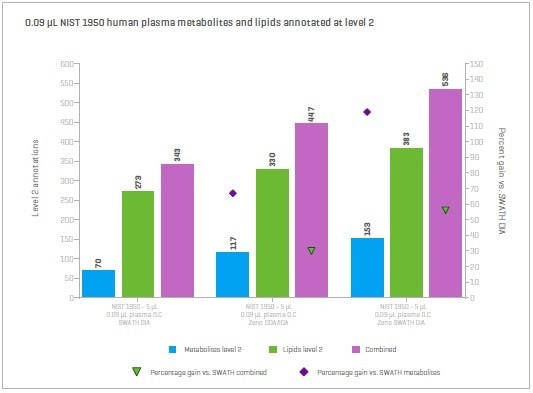
Figure 14. NIST 1950 plasma extracted with a simple methanol protein precipitation and analyzed by reverse-phase chromatography. Shown is a comparison of SWATH DIA, Zeno DDA/IDA and Zeno SWATH DIA. Image Credit: SCIEX
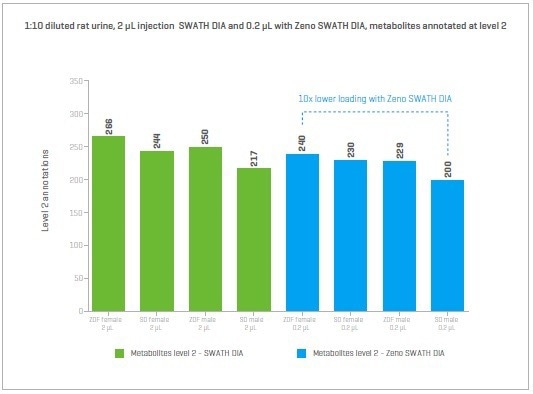
Figure 15. Zucker and Sprague-Dawley rat urine diluted 1 in 10. SWATH DIA loading 2 μL and Zeno SWATH DIA with 0.2 μL (1/10th the volume). Image Credit: SCIEX
In-depth low-level drug metabolite identification
Drug metabolism is a fundamental part of the drug discovery process. Numerous developments have occurred concerning faster and more selective mass spectrometers, but sensitivity in MS/MS mode has not been entirely addressed, particularly when utilizing a data-independent acquisition strategy.
Detection and identification of low-level metabolites is crucial since not all metabolites will ionize similarly, and some of these metabolites may be very relevant for toxicity and pharmacokinetic purposes. Utilization of the Zeno trap with SWATH DIA results in a significant increase in sensitivity.
The impact on detecting metabolites under the conditions tested is a greater than 50% increase in coverage.
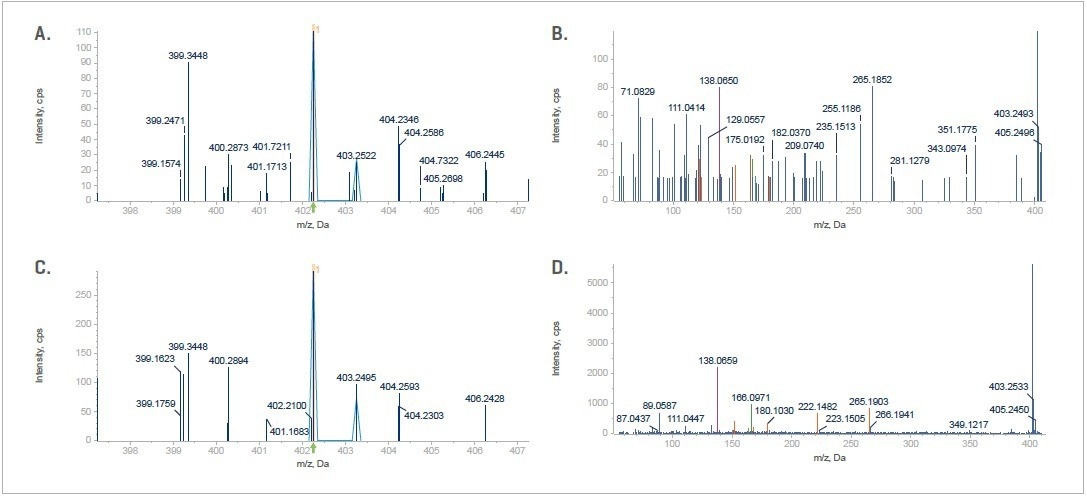
Figure 16. (Top) SWATH DIA for the fifth observed oxidation at the lowest abundance, showing TOF MS data (A) and the MS/MS data (B). (Bottom) Zeno SWATH DIA, for the fifth observed oxidation at the lowest abundance, shows TOF MS data (C) and the MS/MS data (D). Image Credit: SCIEX
Summary
Zeno trap technology has increased the reach of assays for several applications via sensitivity gains delivered through MS/MS duty cycle enhancements. With the Zeno trap, almost >90% duty cycles are now attainable for all ions regardless of charge, mass, or LC flow rate.
This delivers sensitivity gains of 4- to 20-fold without impacting other vital performance specifications, including mass accuracy, dynamic range, speed, and resolution.
The Zeno SWATH DIA workflow now takes advantage of these gains to allow truly widespread and comprehensive analyte coverage at new depths of detection and higher throughput.
With Zeno SWATH DIA, higher sensitivity discovers more lower-level analytes, facilitates more rapid and robust chromatography, and the luxury of utilizing much lower sample amounts.
For proteomics experiments, these gains uncover proteins over a wider and deeper range within plasma, translating to the possibility of single-cell analysis.
Large-scale high throughput proteomics studies are now feasible utilizing robust high-flow chromatography but with significantly less sample consumed.
The new “library-free” approach eliminates the effort of creating an experimental library and instead enables the generation of an in-silico library, making library creation “effort-free”.
Zeno SWATH DIA for metabolomics studies provides lipids and metabolites at deeper levels within the plasma. For drug metabolism studies, new metabolites are uncovered that could be potentially toxic or could help to further the understanding of pharmacokinetics and drug fate.
The reach of Zeno SWATH DIA is far and wide and continues to expand across various applications. The synergy between Zeno technology and the SWATH DIA workflow continues to increase the boundaries of applications needing sensitive and comprehensive, fast high-quality data for analysis.

Image Credit: SCIEX
References and further reading
- Gillet, L.C. et al. Targeted data extraction of the MS/ MS spectra generated by data-independent acquisition: a new concept for consistent and accurate proteome analysis. Mol. Cell. Proteomics 11, O111.016717 (2012).
- Collins, B.C., Hunter, C.L., Liu, Y. et al. Multi-laboratory assessment of reproducibility, qualitative and quantitative performance of SWATH-mass spectrometry. Nat. Commun. 8, 291 (2017).
- Chernushevich, I.V., Merenbloom, S.I., Liu, S., Bloomfield, N. A W-geometry ortho-TOF MS with high resolution and up to 100% duty cycle for MS/MS. J. Am. Soc. Mass Spectrom., 28, 2143-2150 (2017).
- Zeno trap: Defining new levels of sensitivity. (2021) SCIEX white paper, RUO-MKT-19-13373-B.
- Wang, Z. et al. High-throughput proteomics of nanogram-scale samples with Zeno SWATH DIA. BioRxiv preprint.
- Qualitative flexibility combined with quantitative power: Using the ZenoTOF 7600 system, powered by SCIEX OS software (2021) SCIEX technical note, RUO-MKT-02- 13053-B.
- Zeno MS/MS with microflow chromatography powers the Zeno SWATH DIA workflow for more proteins quantified. (2022) SCIEX technical note, RUO-MKT-02-14668-A.
- Demichev, V., Messner, C.B., Vernardis, S.I. et al. DIA-NN: neural networks and interference correction enable deep proteome coverage in high throughput. Nat Methods 17, 41–44 (2020).
- Going library-free for protein identification using Zeno SWATH DIA and in silico-generated spectral libraries. (2022) SCIEX technical note, RUO-MKT-02-14675-A.
- Keshishian, et al. Multiplexed, Quantitative Workflow for Sensitive Biomarker Discovery in Plasma Yields Novel Candidates for Early Myocardial Injury. Mol.Cell. Proteomics Sep;14(9):2375-93 (2015).
- Keshishian, et al. Quantitative, multiplexed workflow for deep analysis of human blood plasma and biomarker discovery by mass spectrometry. Nat. Protoc., Aug;12(8): 1683-1701 (2017).
- High sensitivity protein identification and quantification using Zeno SWATH DIA and nanoflow LC. (2022) SCIEX technical note, RUO-MKT-02-14798-A.
About SCIEX
SCIEX mission is to deliver solutions for the precision detection and quantitation of molecules, empowering their customers to protect and advance the wellness and safety of all.
SCIEX has led the field of mass spectrometry for 50 years. From the moment we launched the first ever commercially successful triple quad in 1981, they have developed groundbreaking technologies and solutions that influence life-changing research and outcomes.
Today, as part of the Danaher family of global life science and technology innovators, they continue to pioneer robust solutions in mass spectrometry and capillary electrophoresis. But they don’t just develop products. It is what they do together with their customers that sets them apart. That’s why thousands of life science experts around the world choose SCIEX to get the answers they can trust to better inform critical decisions. Decisions that positively impact lives.
They proudly stand behind our tagline: The Power of Precision.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.