No modern laboratory is complete without at least one basic microscope, an essential tool for biologists in medical research.
Transmitted light methods have largely been replaced by fluorescent techniques over the last few decades. These newer techniques allow users to confidently identify various cellular structures, including nuclei, mitochondria, cytoskeletal elements, and specifically expressed proteins. Targeted stain-tagged antibodies and low-background labels enhance this process.
Many hours have been spent looking through traditional brightfield or fluorescent microscopes, taking notes, and using manual counters to collect data for studies. But automation, combined with specialized software-driven image analysis, has significantly enhanced microscopy capabilities and kick-started what is now known as high-content analysis (HCA).
HCA is usually associated with 2D cell-based assays (monolayer adherent cell cultures), but it has recently expanded to include more complex biological models that better mimic in vivo conditions. Modern HCA assays may involve 3D biology, such as organoids, spheroids, tumoroids, microtissues, and other cell aggregates, often containing multiple cell types.
HCA can generate various information, from cell counting, proliferation data, and migration, to wound healing metrics, efficacy, toxicology outputs, and so much more. Volumetric data is increasingly sought after and can be produced via confocal imaging and advanced analysis software.
Fast, automated, high-resolution imaging devices capable of collecting data on live, fixed, and 3D samples, paired with powerful new analysis tools such as machine and deep learning, mean that today's HCA platforms are more capable than ever of meeting the demands of complex and meaningful experimental designs.
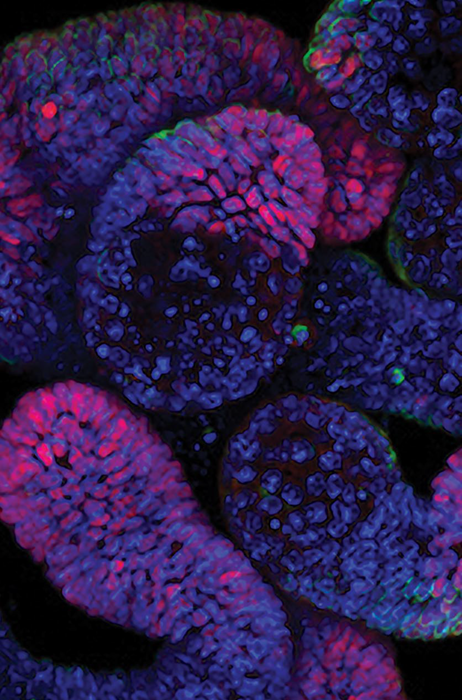
Image Credit: Yokogawa Life Science
Why high-content analysis?
Sometimes referred to as a “Valley of Death,” translational research faces a high attrition rate during drug development because of its challenges, which impact research integrity and hinder medical innovation. However, there are methods to address these issues.
Research integrity depends on the reliability and quality of input data and analysis techniques. Phenotypic screening with high-content analysis enables high-throughput, multiparametric measurement of cellular responses. It quantifies primary target effects while offering an unbiased view of a drug’s mechanism of action.
Additionally, screening compound libraries in cell models that mimic physiological environments can enhance translational research.
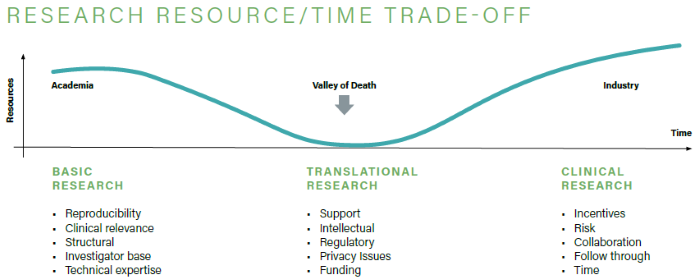
Image Credit: Yokogawa Life Science
Image Credit: Yokogawa Life Science
Confocal and wide-field imaging overview
There are various imaging options available in research, depending on experimental requirements. For instance, wide-field imaging allows researchers to observe confluence and view fluorescent intensities in monolayer samples. Conversely, with its higher resolution and improved signal-to-noise ratio, confocal imaging is ideal for viewing cellular organelles.
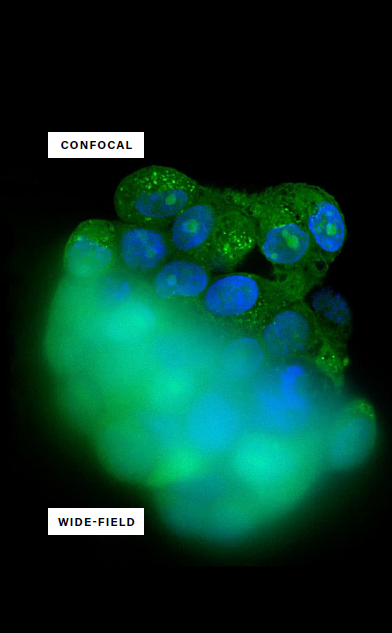
Image Credit: Yokogawa Life Science
Confocal imaging
- Clear images
- Ideal for thick 3D structures
- Suitable for light scattering samples
- Higher cost system
- Limited image capture speed
Confocal imaging employs spatial filtering through pinholes to eliminate out-of-focus light. Only the light in the focused plane reaches the detector, reducing background noise and providing sharp, high-quality images suitable for 3D cell analysis.
Wide-field imaging
- Blurred images
- Suitable for small, dim, or fixed samples
- Not suitable for light scattering samples
- Lower-cost system
- Quick image capture speed
Wide-field imaging captures some out-of-focus light, as well as light in the focal plane, which can result in reduced image quality.
Critical issues in live-cell imaging and analysis
Live-cell imaging enables real-time monitoring of cell functions and behaviors by analyzing intracellular structures and processes from temporal and spatial perspectives.
Live-cell imaging is an essential tool in biological research, offering a practical and affordable way to screen multiple parameters at once. From individual cells to organoids, microtissues, and in vivo models, it allows researchers to explore complex biology in real time across a range of models.
Conventional cell analysis often involves fixing cells with stains or using fluorescence proteins in live cells, requiring assay endpoint optimization, spectral compatibility, and signal-to-noise ratios. Although live-cell imaging has advanced significantly, it continues to face challenges.
Staining
Homogeneous staining is vital for accurate quantification and full culture plate scanning to obtain reliable, statistically relevant data.
Data analysis
Analysing cell structures and quantifying individual cells in monolayers is relatively straightforward, but studying thicker 3D structures such as organoids, cell clumps, spheroids, and microtissues can be more challenging.
Integral incubation
Cultured cells grow heterogeneously regarding size, shape, and distribution. Environmental controls in the imaging chamber regulate temperature, gas concentrations, and humidity to maintain normal cell function.
Image quality vs. speed
High-speed imaging can lead to signal attenuation and poor-quality images, especially when large volumes of images are required from entire culture plates.
Photobleaching and phototoxicity
Prolonged illumination can lead to photobleaching of fluorescent agents and the production of reactive oxygen species, which can harm live cells. Minimizing photobleaching and phototoxicity is essential for accurately mimicking in vivo conditions, necessitating advanced techniques like dual microlensed spinning disks in live-cell imaging.
Case study: 3D model optimization for drug discovery
InSphero AG (Switzerland)
Challenge
InSphero AG, a biotech company focused on in vitro testing, needed to integrate an automated high-speed imaging platform into its drug discovery and safety study framework. The thick and complex 3D in vitro models required a high-content analysis system capable of high-resolution imaging from within the samples and multidimensional analysis.
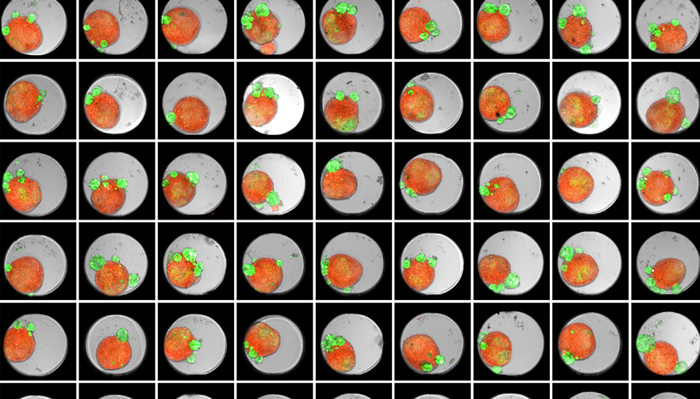
Figure 1. Thumbnail of an Akura 384™ well plate of 3D tumor co-culture microtissues - NCI-N87-GFP (green), NIH-RFP (red), Bright Field (gray). Image Credit: Yokogawa Life Science
Solution
Using the CellVoyager CQ1 benchtop HCA system, which features microlens-enhanced dual Nipkow disk confocal technology, InSphero AG attained high-resolution images of complex microtissue structures from inside thick samples while reducing photobleaching. The automated high-throughput workflow of the CQ1 enabled batch analysis of multiple plates, while the deep learning functions of the CellPathfinder HCA software facilitated spatial and temporal analysis, alongside quantification of various 3D model phenomena.
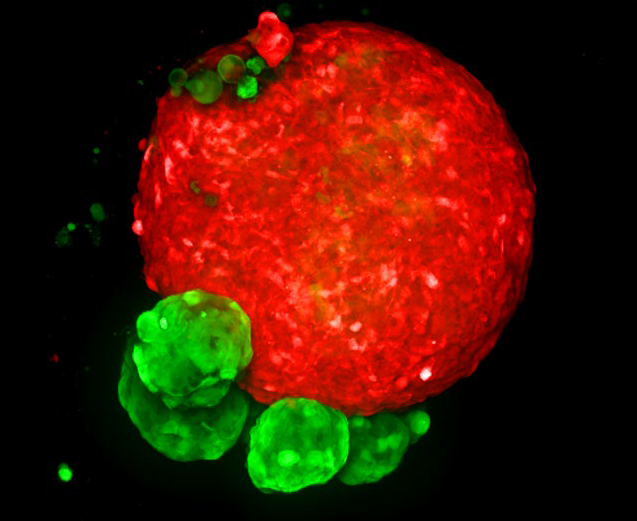
Figure 2. MaxIP image of 3D tumor co-culture microtissue - NCI-N87-GFP (gastric carcinoma; green) and NIH-RFP (murine fibroblast; red). Image Credit: Yokogawa Life Science
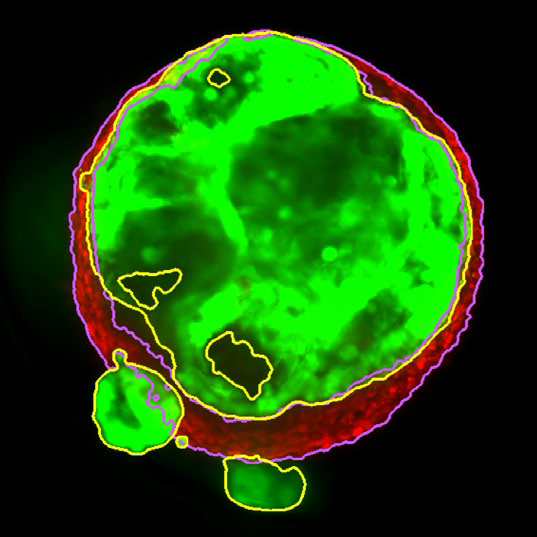
Figure 3. Z slice of the tumor microtissue treated with 0.05 % DMSO - tumor (yellow region) and fibroblast (purple region) are recognized separately in 3D. Image Credit: Yokogawa Life Science
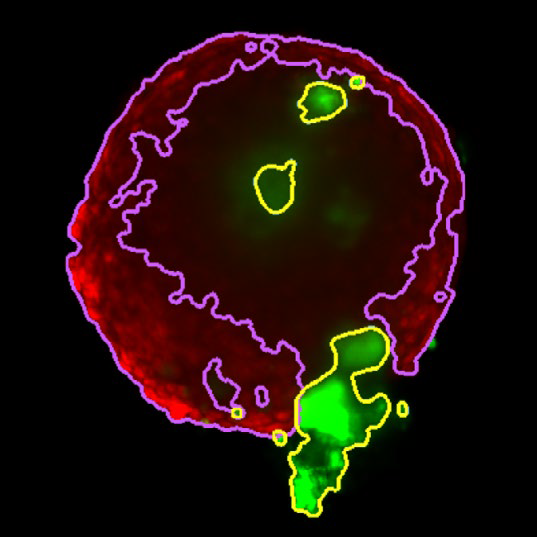
Figure 4. Z slice of the tumor microtissue treated with 5 μM Lapatinib - tumor (yellow region) is decreased significantly compared to the one treated with 0.005 % DMSO. Image Credit: Yokogawa Life Science
Outcome
Tumor spheroids were created by seeding an Akura™ 384 plate with a monodispersed mixture of GFP-expressing NCI-N87 (gastric carcinoma) and RFP-expressing NIH3T3-L1 (murine fibroblast) cells, which spontaneously formed spheroids through scaffold-free self-assembly over several days. After spheroid formation, selected wells containing tumor spheroids were treated with DMSO at 0.05, 0.5, or 5.0 μM Lapatinib for six days. The images were analyzed in 3D, with NCI-N87-GFP (tumor) and NIH-RFP (fibroblast) identified separately, and the volume of each spheroid measured.
This research allowed InSphero AG to accurately quantify pharmacological effects on their 3D models. The fully automated system enhanced multi-condition simultaneous testing, leading to increased workflow efficiency. By implementing the HCA platform, InSphero AG provides seamless solutions, from in vitro 3D models to evaluations.
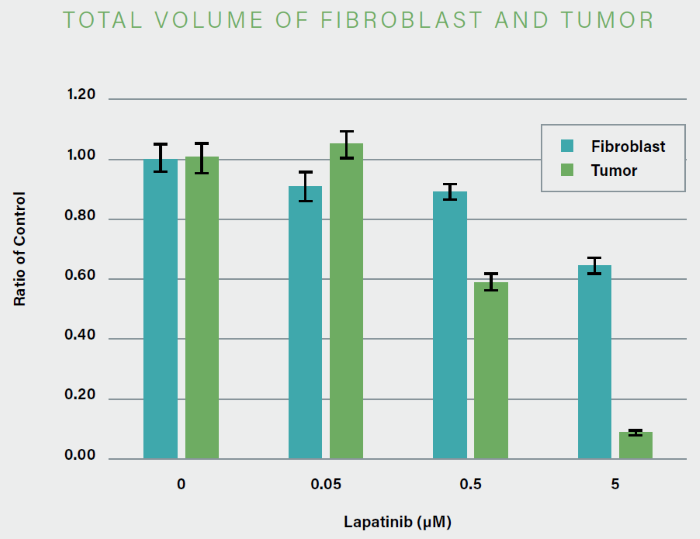
Figure 5. The volume of NCI-N87-GPF (tumor) decreased significantly in a concentration-dependent manner with the addition of Lapatinib. Image Credit: Yokogawa Life Science
About Yokogawa Life Science
We have 30 years of experience in this life science field and will respond to customers' problem-solving with cutting-edge solutions.
Our confocal scanner unit CSU series enables 3D observation of the cells in detail and the dynamics of organelles inside cells. Since the CSU series is capable of high-speed shooting, it is also suitable for observing high-speed life phenomena. In addition, the CSU series is a multi-point confocal method that is extremely gentle to cells, best suited for long-term live cell observation.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net, which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.