Fractional exhaled Nitric Oxide (FeNO) is widely used in both primary and secondary care settings across the world. Many regions recommend incorporating FeNO-guided management into their clinical standards, and resulting FeNO-related guidelines can vary across the UK and other countries. The following explores and compares these approaches.
Guidelines
NICE, BTS, and SIGN guidelines:
In November 2024, the National Institute for Health and Care Excellence (NICE) published the most significant update to UK guidelines in recent years. Jointly released with the British Thoracic Society (BTS) and the Scottish Intercollegiate Guidelines Network (SIGN), the new guidelines looked more closely at asthma diagnosis, monitoring, and chronic asthma management.1
Prior to this, NICE, BTS, and SIGN published their guidelines independently. The newly published guidelines harmonize standards across the board and introduce considerable changes to asthma care techniques, including FeNO testing. This is an objective airway inflammation test that supports asthma diagnosis and management.
ERS guidelines:
In 2022, the European Respiratory Society (ERS) updated its guidelines for the diagnosis of adult asthma. In patients over 18 years of age with suspected asthma, where initial spirometry and bronchodilator reversibility testing have not confirmed a diagnosis, ERS suggests measuring FeNO as part of the diagnostic work-up. This is a conditional recommendation based on moderate-quality evidence.2
DGP guidelines:
The German Respiratory Society (The Deutsche Gesellschaft für Pneumologie und Beatmungsmedizin, DGP) is the largest and oldest medical professional organization for respiratory disorders. Its most recent guidelines on asthma, titled ‘S2K guidelines for specialist diagnosis and therapy of asthma,’ were published in 2023.
In these guidelines, FeNO is identified as a fundamental component of specialist asthma diagnostics.3
ATS guidelines:
In 2011, the American Thoracic Society (ATS) developed guidance on interpreting FeNO testing in adults and children (up to 12 years of age). The most recent American Thoracic Society (ATS) update on using FeNO to guide asthma treatment was published in 2021. It strongly recommends the use of FeNO testing in addition to regular care procedures for the management of asthma in patients.4,5
In response to the ATS guidelines, the American College of Allergy, Asthma and Immunology (ACAAI) and the American Academy of Allergy, Asthma and Immunology (AAAAI), published a joint statement in 2012, saying, “We formally recognize and support the 2011 ATS Clinical Practice Guideline on the Interpretation of Exhaled Nitric Oxide for Clinical Applications”.6
GINA guidelines:7,8
The Global Initiative for Asthma (GINA) works with clinicians, patient groups, and public health bodies around the world to reduce asthma prevalence, morbidity, and mortality. Its 2024 guidelines highlight FeNO as a useful biomarker for both diagnosing and managing the condition, with recommended ppb levels to support diagnosis. While no specific cut-off values are given for children, GINA acknowledges FeNO’s role in guiding treatment decisions.
Reimbursement for FeNO:
Reimbursement policies for FeNO testing differ by country. In England, practices aim to maximize Quality and Outcomes Framework (QoF) points to sustain practice income and cover costs such as the purchase and maintenance of equipment, including FeNO devices.9
Currently, the QoF requirements for asthma diagnosis include spirometry and an additional test, such as FeNO, bronchodilator reversibility, or measures of variability. Recent updates to the BTS/SIGN/NICE guidelines will change the requirements, with practices now expected to carry out at least one objective test to support an asthma diagnosis. For adults, this could include FeNO or blood eosinophil measurement, while for children the first test should be FeNO.
In Germany, FeNO testing is endorsed in the national asthma guidelines. But, it is not reimbursed by Statutory Health Insurance (SHI) in primary care settings. However, in North America, Medicare and Medicare Advantage plans provide reimbursement for FeNO testing if a healthcare professional deems it medically necessary.10
The importance of guidelines:
The use of asthma diagnosis and management guidelines in the application of FeNO is essential worldwide. They help to ensure standardized, evidence-based asthma management tailored to diverse healthcare infrastructures and patient demographics. By empowering medical professionals to make informed decisions, these guidelines enhance the precision of asthma diagnosis and treatment.
Established guidelines help ensure consistent treatment, reduce misdiagnosis, control healthcare costs, and improve patient outcomes. In addition to the countries already discussed, many others, including Japan, Italy, China, France, Mexico, Spain, Malaysia, and Australia, have also developed their own national guidelines.
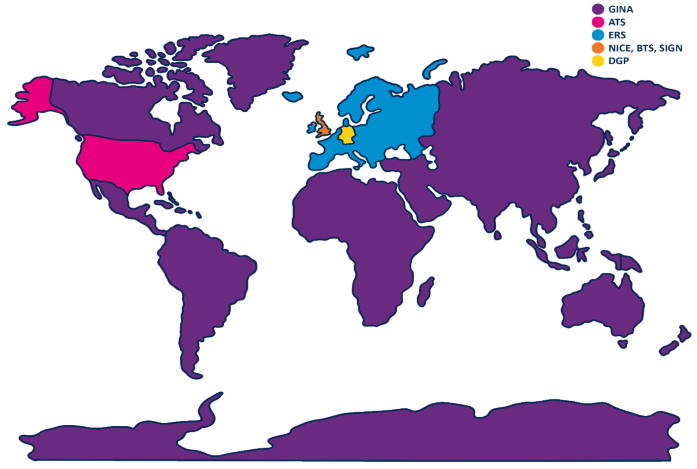
Image Credit: Bedfont® Scientific Ltd
Key takeaways
NICE, BTS, SIGN guidelines:
Diagnosis:
- For adults, a diagnosis of asthma may be supported by FeNO levels of 50 ppb or higher, an increase from the previous NICE guideline threshold of 40 ppb or higher.
- For children, a diagnosis of asthma may be supported by FeNO levels of 35 ppb or higher. This has remained the same as the previous NICE guidelines.
- FeNO testing is recommended as first-line testing in asthma diagnosis for adults and children.
- If the initial test is diagnostic, additional diagnostic testing is not necessary.
Management:
- FeNO testing is recognized as an important tool in asthma management, providing healthcare professionals with valuable information when changing or adjusting asthma therapy.
- FeNO testing is recommended for asthma monitoring in adults.
ERS guidelines:
Diagnosis:
- A cut-off of 40 ppb offers the best balance between sensitivity and specificity. A cut-off of 50 ppb has a high specificity value exceeding 90 %, supporting asthma diagnosis.
- A FeNO value below 40 ppb does not exclude asthma, and, similarly, elevated FeNO levels alone do not confirm a diagnosis.
Management:
- Measuring FeNO is recommended as part of the diagnostic work-up for adults aged 18 years with suspected asthma (conditional recommendation for the test based on moderate-quality evidence).
DGP guidelines:
Diagnosis:
- Low FeNO levels (below 25 ppb in adults and below 20 ppb in children) indicate that eosinophilic inflammation and responsiveness to corticosteroids are less likely.
- Elevated FeNO levels (above 50 ppb in adults and above 35 ppb in children) indicate probable eosinophilic inflammation and, in symptomatic patients, suggest a likely responsiveness to corticosteroids.
Management:
- Patients with high FeNO levels are typically ICS-responsive. High FeNO levels (particularly those above 50 ppb) during ICS therapy, despite clinical stability, indicate that reducing the ICS dose may not be appropriate.
- In children and adolescents, regular FeNO monitoring has proven to be a valuable parameter for predicting asthma relapse following planned ICS discontinuation, even before clinical symptoms appear.
ATS guidelines:
Diagnosis:
- Low FeNO levels (below 25 ppb in adults and below 20 ppb in children) indicate that eosinophilic inflammation and responsiveness to corticosteroids are less likely.
- Intermediate FeNO values (25 to 50 ppb in adults and 20 to 35 ppb in children) should be interpreted with caution and with reference to the clinical context.
- Elevated FeNO levels (above 50 ppb in adults and above 35 ppb in children) indicate probable eosinophilic inflammation and, in symptomatic patients, suggest a likely responsiveness to corticosteroids.
Management:
- FeNO is advantageous and should be employed in addition to usual care.
- FeNO is recommended for monitoring airway inflammation in patients with asthma.
GINA guidelines:
Diagnosis:
- Elevated FeNO levels (above 50 ppb in non-smokers) are moderately associated with eosinophilic airway inflammation.
- FeNO levels ≥ 20 ppb in adults with difficult-to-treat or severe asthma indicate the presence of type two airway inflammation.
Management:
- It is recommended to measure FeNO as an adjunct to diagnostic evaluation in individuals with suspected asthma and to monitor airway inflammation.
FeNO testing with the NObreath®:
Regular FeNO measurements indicate levels of airway inflammation, enabling healthcare professionals to personalize treatment plans by titrating ICS dosing and evaluating patient adherence to treatment.
For more than 48 years, Bedfont® Scientific Limited has specialized in the design and production of breath analysis medical devices. Using cutting-edge technology, Bedfont® provides innovative and affordable medical devices to enhance accessibility and healthcare standards around the world.
Bedfont® manufactures the NObreath® FeNO device, a non-invasive breath testing instrument used to measure airway inflammation for asthma diagnosis and management.

Image Credit: Bedfont® Scientific Ltd
References:
- NICE (2024). Overview | Asthma pathway (BTS, NICE, SIGN) | Guidance | NICE. (online) NICE. Available at: https://www.nice.org.uk/guidance/ng244.
- Louis, R., et al. (2022). European Respiratory Society Guidelines for the Diagnosis of Asthma in Adults. European Respiratory Journal, (online) 60(3). https://doi.org/10.1183/13993003.01585-2021.
- Marek Lommatzsch, Carl-Peter Criée, C.M, C., et al. (2023). S2k-Leitlinie zur fachärztlichen Diagnostik und Therapie von Asthma 2023. Pneumologie. https://doi.org/10.1055/a-2070-2135.
- Dweik, R.A., et al. (2011). An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. American journal of respiratory and critical care medicine, (online) 184(5), pp.602–15. https://doi.org/10.1164/rccm.9120-11ST.
- Khatri, S.B., et al. (2021). Use of Fractional Exhaled Nitric Oxide to Guide the Treatment of Asthma: An Official American Thoracic Society Clinical Practice Guideline. American Journal of Respiratory and Critical Care Medicine, 204(10), pp.e97–e109. https://doi.org/10.1164/rccm.202109-2093st
- Zitt, M. (2017). A Perspective on Fractional Exhaled Nitric Oxide Measurement for Adherence Monitoring. The Journal of Allergy and Clinical Immunology: In Practice, 5(2), pp.523–524. https://doi.org/10.1016/j.jaip.2016.10.014.
- Global Initiative for Asthma (2024). 2024 GINA Main Report. (online) Global Initiative for Asthma - GINA. Available at: https://ginasthma.org/2024-report/.
- Murugesan, N., et al. (2023). Update on the Role of FeNO in Asthma Management. Diagnostics, (online) 13(8), p.1428. https://doi.org/10.3390/diagnostics13081428.
- NHS England (2024). NHS England» Quality and outcomes framework guidance for 2024/25. (online) NHS England. Available at: https://www.england.nhs.uk/publication/quality-and-outcomes-framework-guidance-for-2024-25/.
- Marcin, A. (2022). What You Need to Know About FeNO Testing for Asthma. (online) Healthline. Available at: https://www.healthline.com/health/asthma/feno-test-asthma?c=1285499318383#takeaway (Accessed 23 Jul. 2025).
About Bedfont® Scientific Ltd
Bedfont® Scientific has specialised in the design and manufacture of exhaled breath and gas monitoring instruments since 1976.
For medical gas monitoring, their Medi-Gas Check medical pipeline testing range verifies not only the quantity but also quality of gas administered to patients.
Bedfont's breath analysers include carbon monoxide (CO) monitors such as the Smokerlyzer®, used for smoking cessation, and the ToxCO®, used by emergency services, to diagnose CO poisoning.
The NObreath® FeNO monitor provides accurate analysis of airway inflammation for the control of asthma, and the Gastrolyzer® range aids in the detection of gastrointestinal disorders and food intolerances. Quick and non-invasive, breath analysis is the new blood test.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.