Sponsored Content by Specac LtdReviewed by Maria OsipovaOct 15 2025
Glucagon-like peptide 1 receptor agonists (GLP 1RAs) have transformed the management of type 2 diabetes and weight reduction.
Image Credit: Specac Ltd
The popularity of medications such as liraglutide and semaglutide indicates global demand is rapidly increasing, putting pressure on manufacturers to maintain high quality, purity, and stability for these products.
GLP-1RAs are peptide therapeutics, and so pose characterization problems for quality assurance (QA) and quality control (QC) operations.1
These high-value biopharmaceuticals are susceptible to degradation and aggregation, which can impair therapeutic efficacy and result in undesirable immune responses. Preventing this with structural integrity is critical to safety, efficacy, and regulatory compliance.1-3
Identifying impurities and aggregates in production, particularly during scale-up and manufacturing, requires high sensitivity and specificity. Standard analytical procedures, such as LC-MS or other chromatographic methods, are useful for assessing chemical purity but cannot directly examine molecular conformation.1
This is where infrared spectroscopy, specifically attenuated total reflectance ATR-FTIR spectroscopy, can provide valuable information.
Although its capacity to evaluate protein secondary structure is well established, ATR-FTIR is unexpectedly neglected in biopharmaceutical settings, despite its usefulness in analyzing protein folding and aggregation.4,5,6
This article explores how ATR-FTIR can enhance the assessment of drug identity, stability, and purity in GLP-1RAs. It offers R&D leads, QA/QC scientists, and researchers a practical way to simplify and accelerate analytical workflows in a rapidly evolving field.

Image Credit: Specac Ltd
ATR-FTIR: A powerful lens on peptide structure
When applied to proteins and peptides, FTIR spectroscopy can provide information about secondary structure.
Recent research has discovered that FTIR results correlate well with circular dichroism (CD) spectroscopy, which is extensively used for secondary structure determination but requires specialized equipment and highly purified materials, rendering it incompatible with many common excipients and buffers.6.7
This makes FTIR an appealing, simple, and cost-effective option for peptide therapeutics in pharmaceutical applications where structural fidelity must be closely monitored for safety, efficacy, and compliance.
What makes FTIR ideal for GLP-1RAs?
- Structure-sensitive spectral bands: The amide I (1,700-1,600 cm−1) and amide II (1,600-1,500 cm−1) bands are highly sensitive to peptide backbone conformation. Position and shape variations provide structural details of α-helix, β-sheet, and random coil composition.5,8
- Versatile sample handling: A label-free, non-destructive analytical approach equally effective with aqueous, lyophilized, and crystalline peptide materials.
- Rapid with low input requirements: Scan times are typically under two minutes, and high-quality data can be acquired from a 10 µL sample volume, a significant advantage when limited material is available.
- Ideal for monitoring aggregation: The amide I band distinguishes different β-sheet conformations, making FTIR effective for detecting aggregation.4,9
ATR-FTIR: An ideal approach for biopharmaceuticals and complex matrices
Traditional transmission FTIR requires extensive sample preparation and is impeded by a water absorption peak, which overlaps with the critical amide I band.6,8
To avoid this, peptide and protein analysis commonly uses the ATR sampling technique. An IR laser is internally reflected in a high refractive index crystal, creating an evanescent wave that scans a tiny layer of material (< 5 µm) near the crystal surface.6,8
This reduces the path length, which reduces water interference, and buffer subtraction can further improve the signal.7 Data is subsequently processed to extract secondary structural features, which may include:
- Second-derivative analysis: Separates overlapping amide I features for improved resolution.5
- Fourier self-deconvolution (FSD): An alternate approach for spectral deconvolution.5,10
- Gaussian curve fitting: Allows for structural assignment of amide I/II bands by resolving component peaks (including height, bandwidth, and location).5,11
- Quantitative comparison: Peak regions are assessed quantitatively to determine their structural properties, such as α-helix, β-sheet, and random coil percentages, as well as α-helix/β-sheet ratio.

Image Credit: Specac Ltd
Peptide characterization: Deeper insights and industry evidence
Analytical tools used in pharmaceutical QA/QC workflows must be capable of determining structural integrity. This is especially significant for GLP-1RAs, as aggregation can reduce their bioavailability and jeopardize the drug's therapeutic effectiveness.1,12
While ATR-FTIR can easily detect changes in the secondary structure of peptides and proteins, including contaminants and aggregates,4,13 it is still rarely employed in QA/QC procedures because of its reliance on chromatographic techniques.6
Multiple case studies indicate the importance of closely monitoring structural integrity in GLP-1RAs, as well as the relevance of ATR-FTIR in process development and production workflows.
1. Liraglutide aggregation and pH sensitivity
One study found that liraglutide assembles into micelles of varying sizes depending on pH, and this formation can impact pharmacokinetic parameters. Some adjustments to formulations may be necessary where this occurs.12
- Structural changes were monitored using light scattering and CD spectroscopy techniques.
- ATR-FTIR can detect changes in β-sheet structure during oligomerization without the need for sample preparation activities such as desalting or buffer exchange.
2. Enhanced self-association of lipidated GLP-1RAs
Other researchers looked at the dynamic aggregation behavior of native GLP-1 forms and two lipidated analogues, liraglutide and semaglutide. The researchers used CD spectroscopy, size exclusion chromatography (SEC), and thioflavin T fluorescence to track the oligomerization pathways of lipidated GLP-1RAs.14
- They discovered that the two peptide drugs formed larger and more stable oligomers, highlighting the importance of monitoring aggregation during manufacturing and formulation.
- ATR-FTIR is appropriate for analyzing aggregation kinetics, correlating with increased β-sheet content.
3. Aggregation caused by trace metal exposure
A cross-batch investigation of liraglutide and semaglutide from different manufacturers revealed that trace metal contaminants, particularly Fe2+ and Cu2+, can increase the formation of oligomers, raising concerns regarding the drug's stability and safety.15
- Characterization was conducted through mass spectrometry, thioflavin T fluorescence, and chromatography techniques.
- ATR-FTIR was able to identify aggregation-prone batches by detecting β-sheet content, making it a valuable early screening technique for oligomerization.
These examples demonstrate how ATR-FTIR can provide valuable structural insights for pharmaceutical scientists working in QA/QC, analytical R&D, or formulation development positions without adding significant time or cost. However, there are significant drawbacks that may hinder widespread adoption.
What are the key limitations of peptide ATR-FTIR?
- This approach offers lower structural resolution compared to NMR but is faster, cheaper, and easier to employ in QA/QC laboratories.
- Expertise in spectral deconvolution is necessary to obtain structural information.
- Water absorption interference is typically mitigated using a matching buffer for background scans, even during real-time in situ measurements.7
- Sensitivity limit for signal quality < 1.0 mg/mL.8,16
Overcoming sensitivity constraints with ConcentratIR2
While the above problems are usually acceptable, ATR-FTIR requires protein samples in the mg/mL range,8 with a lower limit of ~0.7 mg/mL for antibodies.16 However, some completed therapeutic formulations contain proteins in far lower concentrations, making analysis more difficult.
High-performance ATR accessories, such as the Harrick ConcentratIR2™ Multiple Reflection ATR Accessory, with a 23-bounce silicon ATR crystal, can significantly improve signal-to-noise ratio and overcome this constraint.
Using a commercial FTIR spectrometer, resolvable amide I/II bands were produced at low concentrations for two injectable peptide medicines, ziconotide (25 µg/mL) and calcitonin (33 µg/mL) (Figure 1).
At a greater concentration (5 mg/mL), the peptide medication leuprolide acetate produced high-quality spectra in the amide I/II region.
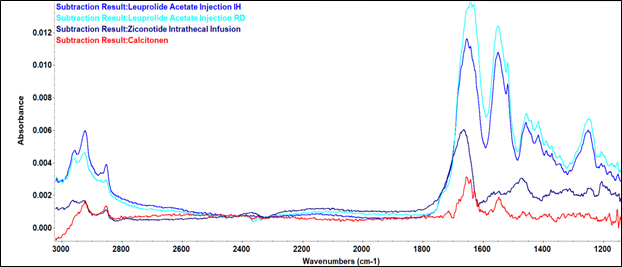
Figure 1. Post-Subtraction Infrared Spectra of Peptide Therapeutics Showing Amide I and II Bands Using the ConcentratIR2 ATR Accessory. Image Credit: Specac Ltd
The ConcentratIR2™ can analyze secondary structure at low concentrations, demonstrating its versatility. This is beneficial for biopharmaceuticals like GLP-1RAs that require monitoring at µg/mL levels.
Implications and future directions
Because of its clinical success, GLP-1RAs have emerged as important therapies for diabetes and obesity. As demand rises, pharmaceutical teams must maintain consistent quality, structural stability, and batch-to-batch repeatability while expanding production and entering new markets.
This results in a renewed emphasis on dependable, scalable, and orthogonal QA/QC frameworks for evaluating purity and stability. ATR-FTIR, as a quick and cost-effective technology, is well-suited to enter this arena, particularly for detecting aggregates that can impair therapeutic efficacy and potentially trigger immunogenicity.1,3
ATR-FTIR: A strategic complement, not a replacement
Rather than replacing other methods, ATR-FTIR provides complementary analytical insights that pharmaceutical teams can apply without incurring significant resource costs. When combined with products like the ConcentratIR2™ ATR Accessory, it provides strategic opportunities to:
- Detect early indicators of aggregation or formulation instability.
- Identify structural anomalies before applying advanced biophysical approaches.
- Relate structural data to batch variance, manufacturing size, and storage circumstances.
- Use specification-based alarms for atypical secondary structure profiles, such as during in-process controls (IPCs).
- Improve release testing, particularly for generic GLP-1RAs.
A practitioner’s perspective
For QA/QC managers, ATR-FTIR offers:
- Speed to decision: Rapid scan times give structural insights in minutes.
- Scalability: Amenable to high-throughput implementation.6
- Affordability: Lower barrier to entry than many biopharmaceutical analytical tools.
- Simplicity: Reduced training and operational overheads compared with LC-MS, SEC, or CD spectroscopy setups.

Image Credit: Specac Ltd
Bringing ATR-FTIR into the peptide QA/QC mainstream
As GLP-1RA production develops globally, it is critical to maintain batch uniformity. Although frequently disregarded in pharmaceutical QA/QC settings, ATR-FTIR is well positioned to deliver rapid, label-free structural knowledge that complements traditional test approaches.
It is especially useful for evaluating important quality aspects, including secondary structure and aggregation, at a low cost.
The ConcentratIR2™ ATR Accessory enhances ATR-FTIR to identify GLP-1RAs and other therapeutic peptides at trace levels (µg/mL), making it more suitable for modern QA/QC procedures.
If you are an R&D leader, QA/QC manager, or peptide therapies researcher, consider ATR-FTIR as a forward-thinking tool to improve your structural analytics.
References
- The Analytical Scientist. (2025). GLP-1 Receptor Agonists. (online) Available at: https://www.theanalyticalscientist.com/issues/2025/articles/may/analysis-and-characterization-of-glp-1-peptides/.
- De, A.S., et al. (2025). Immunogenicity of Generic Peptide Impurities: Current Orthogonal Approaches. Pharmaceutical Research, (online) 42(5), pp.805–818. https://doi.org/10.1007/s11095-025-03843-1. .
- L. Wu, in Peptide Therapeutics: Strategy and Tactics for Chemistry, Manufacturing and Controls, ed. V. Srivastava, 2019, ch. 1, pp. 1–30.
- Tintor, Đ., et al. (2024). Gaining insight into protein structure via ATR-FTIR spectroscopy. Vibrational Spectroscopy, 134, p.103726. https://doi.org/10.1016/j.vibspec.2024.103726.
- Yang, H., et al. (2015). Obtaining information about protein secondary structures in aqueous solution using Fourier transform IR spectroscopy. Nature Protocols, 10(3), pp.382–396. https://doi.org/10.1038/nprot.2015.024.
- Tiernan, H., Byrne, B. and Kazarian, S.G. (2020). ATR-FTIR spectroscopy and spectroscopic imaging for the analysis of biopharmaceuticals. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, (online) 241, p.118636. https://doi.org/10.1016/j.saa.2020.118636.
- Zhao, J., et al. (2021). Real-time in-situ quantification of protein secondary structures in aqueous solution based on ATR-FTIR subtraction spectrum. Biochemical Engineering Journal, 176, p.108225. https://doi.org/10.1016/j.bej.2021.108225.
- Barth, A. (2007). Infrared spectroscopy of proteins. Biochimica et Biophysica Acta (BBA) - Bioenergetics, 1767(9), pp.1073–1101. https://doi.org/10.1016/j.bbabio.2007.06.004.
- Shivu, B., et al. (2013). Distinct β-Sheet Structure in Protein Aggregates Determined by ATR–FTIR Spectroscopy. Biochemistry, 52(31), pp.5176–5183. https://doi.org/10.1021/bi400625v.
- KONG, J. and YU, S. (2007). Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Biochimica et Biophysica Sinica, 39(8), pp.549–559. https://doi.org/10.1111/j.1745-7270.2007.00320.x.
- Titus, J., et al. (2017). Protein secondary structure analysis of dried blood serum using infrared spectroscopy to identify markers for colitis screening. Journal of Biophotonics, 11(3). https://doi.org/10.1002/jbio.201700057.
- Wang, Y., et al. (2015). Transformation of Oligomers of Lipidated Peptide Induced by Change in pH. Molecular Pharmaceutics, 12(2), pp.411–419. https://doi.org/10.1021/mp500519s.
- Kendrick, B.S., et al. (2020). Determining Spectroscopic Quantitation Limits for Misfolded Structures. Journal of pharmaceutical sciences, (online) 109(1), pp.933–936. https://doi.org/10.1016/j.xphs.2019.09.004.
- Přáda Brichtová, E., et al. (2025). Effect of Lipidation on the Structure, Oligomerization, and Aggregation of Glucagon-like Peptide 1. Bioconjugate Chemistry. https://doi.org/10.1021/acs.bioconjchem.4c00484.
- Staby, A., et al. (2020). Influence of Production Process and Scale on Quality of Polypeptide Drugs: a Case Study on GLP-1 Analogs. Pharmaceutical Research, 37(7). https://doi.org/10.1007/s11095-020-02817-9.
- Boulet-Audet, M., Kazarian, S.G. and Byrne, B. (2016). In-column ATR-FTIR spectroscopy to monitor affinity chromatography purification of monoclonal antibodies. Scientific Reports, 6(1). https://doi.org/10.1038/srep30526.
About Specac Ltd
Specac manufactures an extensive range of FTIR Accessory, IR Polarizer, and Pellet Press Products for Atomic and Molecular Spectroscopy.
These products include ATR Accessories, Specular Reflectance Accessories, Diffuse Reflectance Accessories, Liquid Transmission and Gas Transmission Cells, as well as Infrared and Terahertz Wire Grid Polarizers, Bench-Top Hydraulic Presses, KBr Pellet Presses, XRF Pellet Presses, Thin Film Making Kits, and Evacuable Pellet Dies.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.