Sponsored Content by Specac LtdReviewed by Maria OsipovaOct 15 2025
Introduction
Polyethyleneimines (PEIs) are extensively researched as non-viral vectors for gene and medication delivery because of their capacity to condense nucleic acids into nanoparticles known as polyplexes.1
Compared to viral vectors, PEIs have lower immunogenicity, higher loading capacity, and lower production costs.2
However, their clinical translation has been hampered due to low transfection efficiencies.3
The type of polyplex formation, which regulates cellular absorption and intracellular release, is an important element in managing transfection.
Effective delivery systems require polyplexes that are sturdy enough to avoid premature nucleic acid release, while remaining weak enough to release the payload at the appropriate time and position within the cell.4
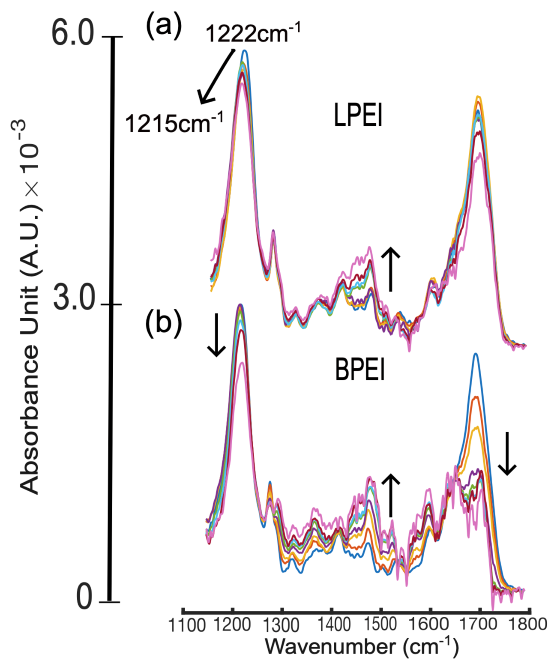
Figure 1. ATR-FTIR spectra of DNA in the presence of LPEI (a) and BPEI (b) in phosphate buffer (pH = 5.0). The black arrow indicates increasing N/P ratio from 0-3. Image Credit: Specac Ltd
Understanding the intermolecular interactions and conformational changes involved in polyplex synthesis and stability is critical for enhancing PEI-based delivery platforms. According to Mustafa et al., attenuated total reflectance infrared (ATR-IR) spectroscopy is ideal for this application.5
It reveals molecular-level information about DNA structure and binding via vibrational modes of the phosphate backbone and nucleobases. This article shows how ATR-FTIR can be utilized to characterize polyplex production using linear and branching PEI architectures.
Methods
Mustafa et al. provide a detailed discussion of their procedures. ATR-FTIR measurements were taken using a commercially available FTIR spectrometer with a Harrick ConcentratIR2™ multiple reflection silicon ATR attachment.5
Two PEI structures were investigated: linear (LPEI, 15 kDa) and branching (BPEI, 10 kDa). Polyplexes were generated at N/P ratios ranging from zero to three in 0.01 M phosphate buffer (pH 7.4).
The final concentrations were three millimolar LPEI, five millimolar BPEI, and one millimolar DNA. Before measurements were taken, the samples were incubated for one hour at room temperature.
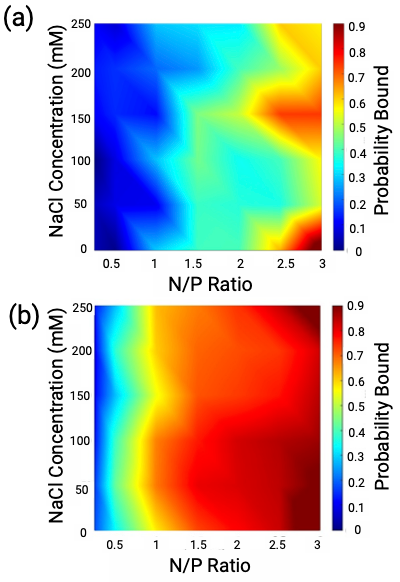
Figure 2. Diagrams showing the probability of bound DNA by polymers obtained from the ATR-FTIR spectra of LPEI and BPEI polyplexes in the presence of varying NaCl concentrations (50-250 mM). Image Credit: Specac Ltd
To confirm the spectrum interpretation, additional samples were generated at increasing sodium chloride concentrations (50-250 mM) to test for electrostatic interactions. All spectra were background removed, baseline corrected, and normalized.
Results and discussion
Figure 1 shows ATR-FTIR spectra of DNA with increasing N/P ratios for LPEI and BPEI. With LPEI, the asymmetric phosphate stretching band at 1222 cm-1 shows a noticeable frequency shift as N/P rises, showing strong electrostatic binding to the DNA backbone.
This frequency shift also signals a conformational change in the DNA structure, from B to Z. Moderate alterations were found in the nucleobase ring stretching region (1400-1500 cm-1) and carbonyl band (1680 cm-1). These modifications imply that LPEI interacts weakly with DNA nucleobases.
The spectra of BPEI-based polyplexes exhibited no shift at 1222 cm-1, but a considerable intensity decrease and splitting of the 1680 cm-1 band, indicating interactions with nucleobases via intercalation, without major disruption of the phosphate backbone.
Salt screening assays supported these interpretations. Figure 2 shows that increasing NaCl reduced LPEI binding by around 70 %, consistent with electrostatic interactions. At the greatest salt concentrations, BPEI binding decreased by just 20-30 %, indicating that interactions with DNA nucleobases primarily aid binding.
Conclusion
This study shows that ATR-FTIR with the ConcentratIR2 accessory may successfully distinguish between binding modes in PEI-DNA polyplexes.
The 1222 cm-1 phosphate band detects electrostatic interactions between LPEI and DNA, while the 1680 cm-1 band indicates BPEI intercalation with nucleobases. These findings serve as a foundation for developing more successful non-viral gene delivery technologies.
References
- Degors, I.M.S., et al. (2019). Carriers Break Barriers in Drug Delivery: Endocytosis and Endosomal Escape of Gene Delivery Vectors. Accounts of Chemical Research, 52(7), pp.1750–1760. https://doi.org/10.1021/acs.accounts.9b00177.
- Puhl, D.L., D’Amato, A.R. and Gilbert, R.J. (2019). Challenges of gene delivery to the central nervous system and the growing use of biomaterial vectors. Brain Research Bulletin, (online) 150, pp.216–230. https://doi.org/10.1016/j.brainresbull.2019.05.024.
- Casper, J., et al. (2023). Polyethylenimine (PEI) in gene therapy: Current status and clinical applications. Journal of Controlled Release, (online) 362, pp.667–691. https://doi.org/10.1016/j.jconrel.2023.09.001.
- Kumar, R., et al. (2021). Polymeric Delivery of Therapeutic Nucleic Acids. Chemical Reviews, 121(18), pp.11527–11652. https://doi.org/10.1021/acs.chemrev.0c00997.
- Mustafa, R., et al. (2025). Revealing Two Distinct Molecular Binding Modes in Polyethyleneimine-DNA Polyplexes using Infrared Spectroscopy. Soft Matter. (online) https://doi.org/10.1039/d5sm00213c.
About Specac Ltd
Specac is a global spectroscopy accessory and sample preparation business with decades of experience in designing state-of-the-art solutions for molecular and atomic spectroscopies, including FTIR, NIR, UV-Vis, XRF, and Raman. Applications range from analysis of raw materials in pharmaceutical manufacturing through to cutting-edge chemical diagnostics. Our dedicated Applications Team can support customers in selecting and evaluating products for your application, using our dedicated on-site spectroscopy testing facilities.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.