hVIVO has recently used its expertise in challenge studies to support a large outpatient vaccine trial in healthy volunteers.
Study requirements and objectives
A biotech company based in the United States aimed to undertake a large-scale outpatient vaccine study involving approximately 5,000 healthy participants in the US and UK.
hVIVO’s goal as part of this work was to recruit around 1,000 participants in the UK prior to the beginning of the influenza season. A 26-week influenza-like illness follow-up was then performed.
Dosing was to be completed before the influenza season began, making use of the later onset of the influenza season in the UK versus the US to better align with the client’s timelines.
The US-based biotech company was already operating in the US. hVIVO was contracted as the exclusive UK-based site due to its potential for improved efficiency in both operations and time.
Timelines for this study were challenging. Recruitment was scheduled to start in September 2024, with hVIVO promptly completing the feasibility assessment as soon as the protocol was delivered in early June 2024.
Operational execution
The study’s success is attributed to the close collaboration between the sponsor, internal hVIVO teams (including laboratory staff, pharmacy, recruitment teams, and site personnel), and an external team of clinical research associates (CRAs) that were embedded with the sponsor.
These CRAs worked on site to ensure close cooperation between hVIVO staff and sponsors. Flexibility was essential, prompting the team to optimize clinic hours (including weekends) and deliver extended dosing sessions in order to maximize the number of dosed participants.
Clinical process optimization
The team sought to dose 20 participants per session, but constant adjustments were needed to optimize efficiency in response to the unpredictable turnout of participants.
hVIVO implemented three dosing sessions per day (for example, 7:30 AM, 10:30 AM, and 1:30 PM) to accommodate varying numbers of participants, with each session staffed by at least seven clinical study support (CSS) personnel, five doctors, three nurses, and one phlebotomist.
Dry runs were conducted to optimize facility utilization prior to commencing the actual study. This proved crucial in ensuring a smooth operational flow on treatment days. The clinical administration team was required to effectively manage casebooks, paperwork filing, consent verification, and coordinate triggered assessments in instances where participants experienced acute respiratory infections.
The enrolment team was responsible for coordinating follow-up visits to maintain participant engagement, while the data management team ensured that all source data was transferred into the sponsor’s electronic data capture system.
Image Credit: hVIVO
Screening and dosing process flow
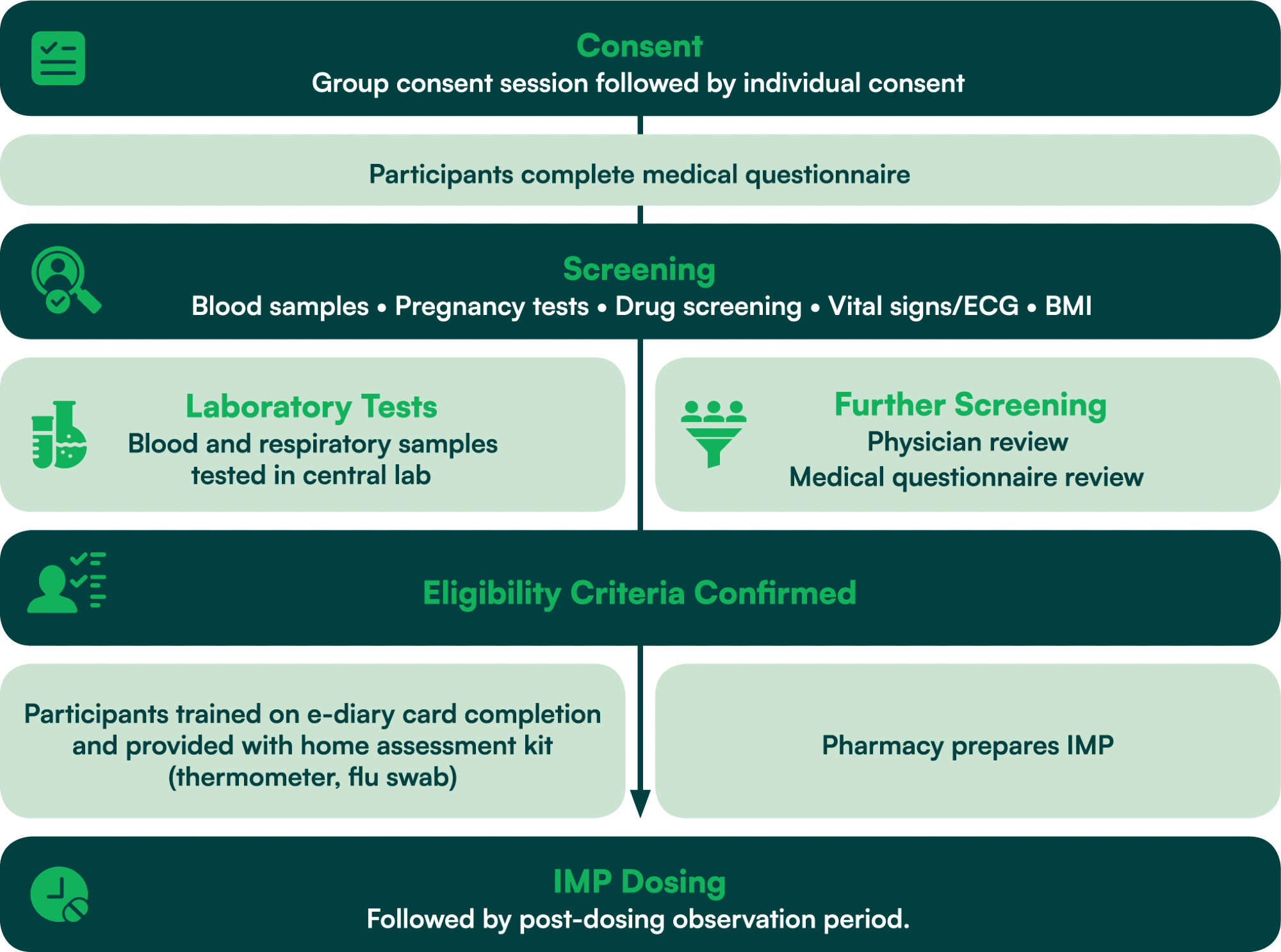
Image Credit: hVIVO
Study schedule flowchart
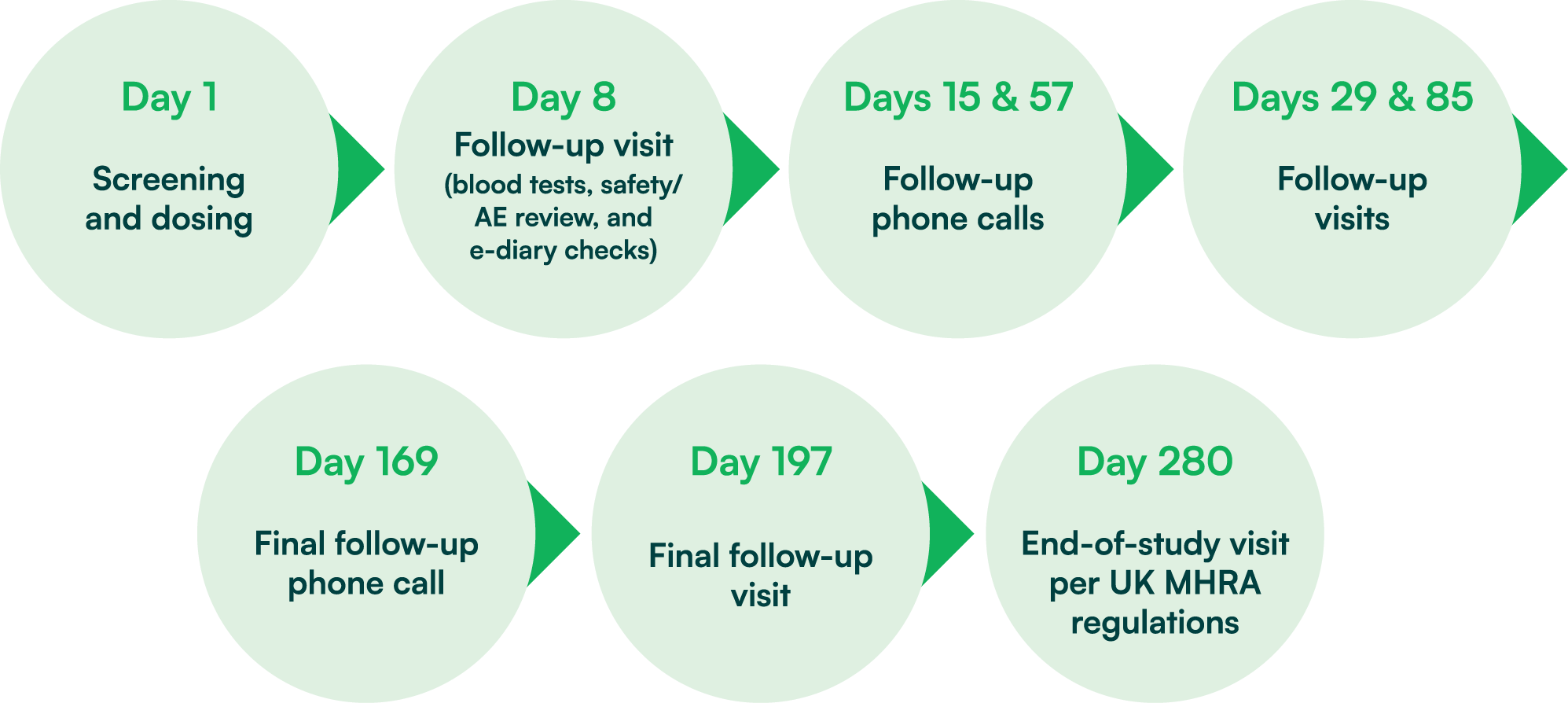
Image Credit: hVIVO
Challenges and solutions
hVIVO’s proactive approach and expert knowledge enabled potential issues to be resolved before they adversely affected the study's success.
These issues included unpredictable participant turnout, which could range from as few as 1 to 2 participants to as many as 43 participants per session. Real-time staffing adjustments were necessary to manage this effectively.
Dry runs also helped to ensure efficient use of space, manage the large workflow, and minimize overlap between screening and dosing teams. Snacks were provided to help keep participants engaged, mitigate waiting times, and prevent participant dropouts.
hVIVO’s team of specialist virology scientists, its facilities, and its versatile services proved invaluable in running this vaccine study. The company’s expertise in collecting, managing, and analyzing specialist virology samples was key to ensuring the acquisition of robust and comprehensive study data.
Scientific advice was provided on updating laboratory protocols at all study sites to ensure optimal sample preservation, and guidance was offered on the IMP and blinding process.
hVIVO’s integrated clinical trial site and laboratory services allowed for a seamless and efficient process throughout the entirety of the study.
Its extensive experience in managing challenge trials also allowed data to be modeled, with resource allocation, necessary staffing levels, and recruitment run rates all predicted to effectively deliver on expectations and proactively address challenges.
Processes were also monitored, measured, and adjusted where necessary, allowing the delivery of highly agile services.
Key metrics and achievements
hVIVO screened an average of 170 participants and dosed approximately 130 participants per week. More than 1,100 participants were screened in just over six weeks, with 817 participants (74 %) successfully dosed, with follow-up currently underway.
Approximately 40 % of participants who failed screening were excluded due to self-reported medical history, 25 % due to factors such as out-of-range ECG, body mass index, or vital signs, and 15 % due to positive drug tests.
Conclusion
hVIVO’s extensive expertise in virology and clinical operations allowed this trial to be executed seamlessly, despite the significant challenges associated with the project.
hVIVO successfully dosed 817 subjects in just over six weeks, representing an extraordinary achievement considering the unpredictable nature of participant turnout and the study’s complexity.
The team’s ability to rapidly optimize resources, adapt to fluctuating numbers, and ensure high-quality clinical execution was fundamental to its success in meeting the trial’s ambitious aims.
This outcome successfully showcases hVIVO’s position as a premier clinical research site in the UK, highlighting its capacity to recruit for and effectively manage large-scale vaccine studies efficiently.
The capacity to screen, dose, and follow up on such a significant number of participants within a limited timescale is a testament to hVIVO’s agile methodology, operational excellence, and deep scientific expertise.
The company’s combination of expert scientists, clinicians, and clinical trial staff, its integrated site and lab facilities, and its impressive participant capacity can help to expedite the development of a wide range of vaccine and antiviral products.

Image Credit: hVIVO
Acknowledgments
Produced from materials originally authored by hVIVO.