Coronavirus disease 2019 (COVID-19), caused by the rapid outbreak of severe acute respiratory disease coronavirus-2 (SARS-CoV-2), has claimed more than 5.5 million lives worldwide. Scientists have estimated that herd immunity could be reached if 70%-80% of the world population is vaccinated. Still, according to the latest report, to date, only 59.2% of the world population has received at least one dose of a COVID-19 vaccine. However, only 8.9% of people in low-income countries have received at least one dose.
The vaccination process has been slowed down owing to multiple factors that include vaccine hesitancy, shortage of essential components required to develop vaccines, and resource-poor settings for vaccine development or vaccination, which is common in low-income countries. Also, the requirement of cold chain storage for vaccine distribution has delayed vaccination activities.
As vaccinating the entire world's population is time-consuming, scientists expressed the urgency in developing effective, safe, easy-to-produce, and economical prophylaxis to prevent or reduce the risk of COVID-19 infection in the unvaccinated population.
Additionally, several SARS-CoV-2 variants, such as the Delta and Omicron strain, are more contagious than the original strain and can evade vaccine-induced immune protection. Also, among the approved or authorized therapeutics, seven out of eight monoclonal antibodies could not neutralize the Omicron variant.
These facts indicate the urgent need for an alternative approach that could aid in containing the COVID-19 pandemic.
 Study: Egg-derived anti-SARS-CoV-2 immunoglobulin Y (IgY) with broad variant activity as intranasal prophylaxis against COVID-19: preclinical studies and randomized controlled phase 1 clinical trial. Image Credit: NIAID
Study: Egg-derived anti-SARS-CoV-2 immunoglobulin Y (IgY) with broad variant activity as intranasal prophylaxis against COVID-19: preclinical studies and randomized controlled phase 1 clinical trial. Image Credit: NIAID

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Intranasal Antibody Prophylaxis and SARS-CoV-2
The main entry route of SARS-CoV-2 is the nasal mucosa, which has increased levels of angiotensin-converting enzyme 2 (ACE2) receptor. The spike protein of SARS-CoV-2 binds with host ACE2 to establish infection. Hence, the nasal mucosa could act as a critical barrier and reduce viral entry.
Anti-SARS-CoV-2 antibodies introduced to nasal epithelial surfaces could also block the lateral viral motility and agglutinate viral particles. Therefore, intranasally administered antibodies could play an essential role in preventing and transmitting SARS-CoV-2 infection.
In the past, intranasal antibody prophylaxis has proved to be effective against multiple respiratory tract viruses and other pathogens in humans and veterinary applications. Hence, researchers believe that introduction of anti-SARS-CoV-2 antibodies in the nasal mucosa could effectively protect unvaccinated individuals and reduce viral transmission.
Egg-Derived Intranasal Prophylaxis against COVID-19
A new study, posted to the medRxiv* preprint server, has focussed on the development of intranasal prophylaxis against SARS-CoV-2 infection.
In this study, researchers used the receptor-binding domain (RBD) of the spike protein of SARS-CoV-2 as the immunogen. They immunized egg-laying hens to raise anti-SARS-CoV-2 polyclonal antibodies. This method of raising antibodies has been reported to be rapid, low cost, and yielding a high volume of antibodies. Scientists have also analyzed the neutralization efficacy of the antibodies against currently circulating viral variants.
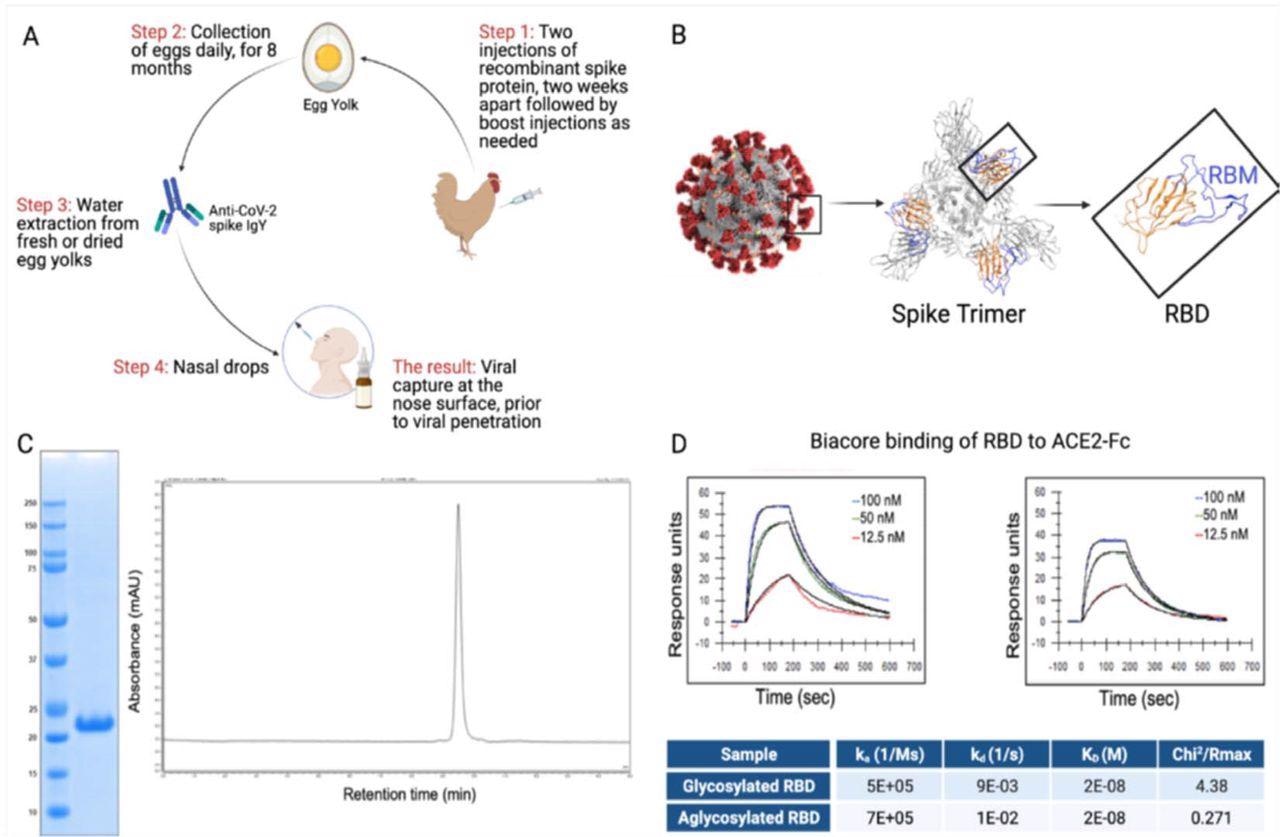
RBD and IgY preparation. (A) Workflow of the study. IgY preparation for intranasal drops as antiviral prophylaxis. (B) Cell-free expressed RBD derived from the Spike protein on the viral envelope of SARS-CoV-2. (C) Characterization of the recombinant protein RBD by ELISA and HPLC. (D) Determination of the affinity of the cell-free expressed RBD (amino acids 328-533) and mammalian-expressed full-length S1 to the hACE2 using Biacore.
A phase 1 study revealed that these antibodies are safe and tolerated well when introduced to healthy humans as an intranasal drop. Additionally, researchers also studied the pharmacokinetics of anti-SARS-CoV-2 RBD immunoglobulin Y (IgY).
The authors have reported that the IgY antibody is concentrated in commercial hens' eggs within 2-3 weeks of vaccination. The levels of antibodies were estimated to be 50-100 mg/egg. This yield was enhanced fivefold when scientists used specific pathogen-free (SPF) hens.
Initially, researchers analyzed the safety profile of IgY, raised in SPF hen, by intranasally administering drops (4 mg/day) of antibodies in rats for 28 days.
This good laboratory practice (GLP) study found no evidence of any toxicity or innate inflammatory response upon systemic exposure to IgY. Similarly, anti-SARS-CoV-2 RBD IgY was intranasally given to healthy adult participants at a single ascending dose for fourteen days, and all participants revealed a highly safe and tolerable profile.
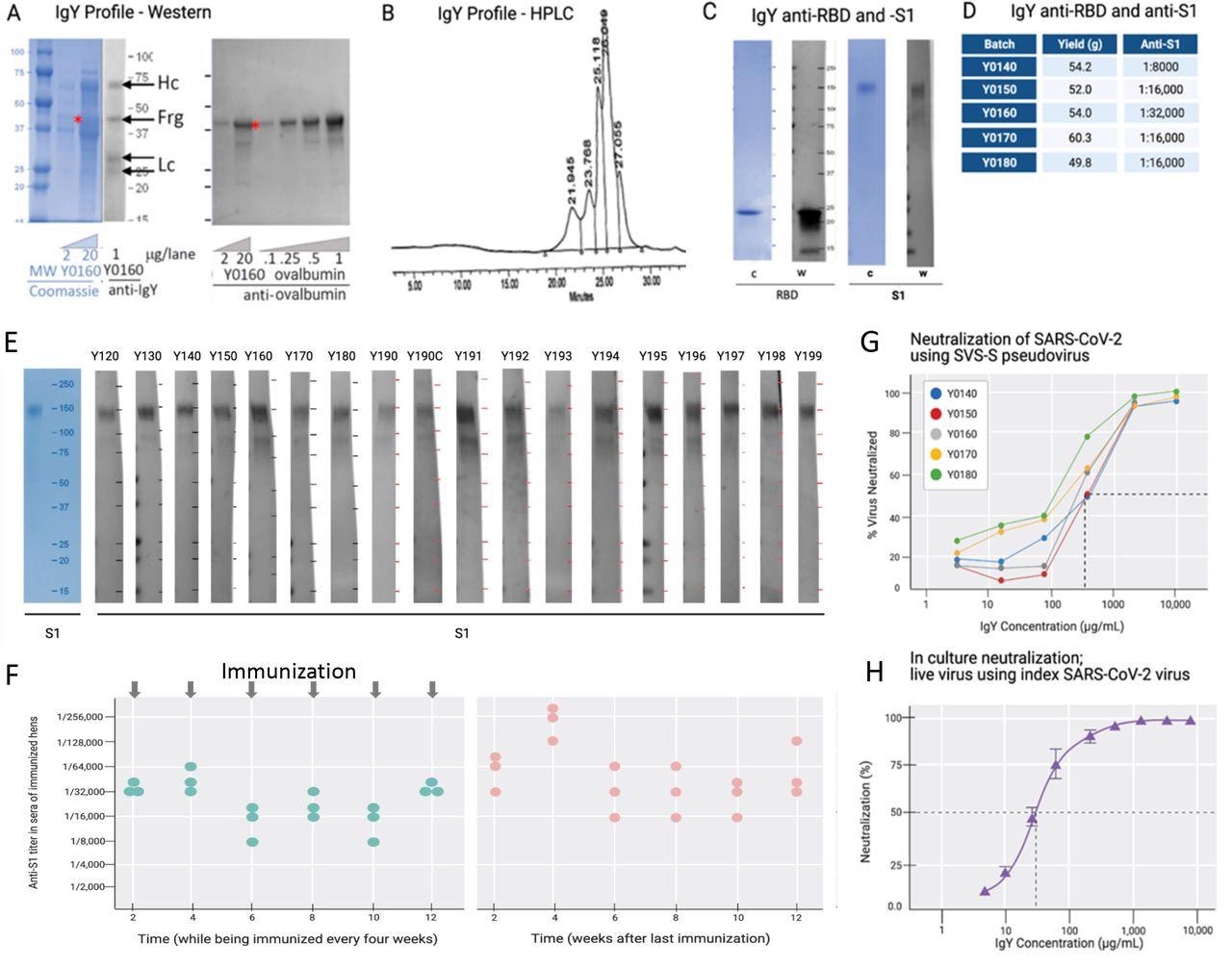
IgY purification and characterization. (A) Western blot analysis of the IgY preparation. (B) HPLC profile of the IgY preparation. (C) Western blot analysis of anti-SARS- CoV-2 IgY against RBD fragment and full S1 recombinant protein. (D) IgY yield for various batches derived from 100 eggs each. (E) Western blot data of different lots of anti-SARS-CoV-2 RBD IgY (Y0120-Y0199). Pools of 100 eggs laid by 9 hens over 2 weeks were used for each pool of IgY preparation between May 2020 and March 2021. IgY lot samples were diluted 1:500 followed by a 1:3000 dilution of rabbit anti-IgY HRP conjugate. First left lane shows the Coomassie stain of the same gels. (F) Time-dependent ELISA titers of sera from 3 individual hens following continual immunization (left); arrows indicate immunization timing. Time-dependent ELISA titer of 3 hens after immunization was stopped for up to 12 weeks (right). (H) Neutralization of pseudovirus SARS-CoV-2 by various lots of anti-SARS-CoV-2 RBD IgY (conducted at RetroVirox). (I) Neutralization of live index SARS-CoV-2 virus by anti-SARS- CoV-2 RBD IgY (Y0180, conducted at USAMRIID).
Also, none of the participants who received intranasal anti-SARS-CoV-2 IgY in the multiple-dose phase showed measurable levels of anti-SARS-CoV-2 RBD IgY in their sera. This suggests the lack of systemic absorption after the internasal introduction of IgY.
Importantly, no evidence of systemic inflammatory immune response was triggered by this internasal treatment with anti-SARS-CoV-2 RBD IgY in humans. Additionally, researchers found no detectable elevation of cytokines in human sera.
Scientists designed the anti-SARS-CoV-2 RBD IgY in such a manner that it is capable of capturing and immobilizing the SARS-CoV-2 virus present in the nasal mucosa. This can restrict the virus from binding to and spreading across the nasal mucosa and prevent the transmission of the virus from one individual to another.
![Common variants of SARS-CoV-2 and anti-SARS-CoV-2 IgY interaction with them. (A) A scheme depicting locations of mutated amino acids in Alpha through Mu variants of SARS-CoV-2, focusing on the RBD domain only. Each color bar indicates the amino acid in the index virus that was mutated in the variant. (B) Spike protein of SARS-CoV-2 Alpha, Beta, Delta, and Omicron are shown from left to right. Molecular Operating Environment was used to create the figure [31]. The location of mutations in the structure of the S protein trimer of SARS-CoV-2 (PDB ID: 7A98) for 4 of the common variants are indicated in red and glycosylation sites are indicated in pink throughout the S protein. Blue ribbon indicates RBD (amino acids 328-533) and the orange ribbon indicates receptor binding motif (amino acids 437- 508). (C) Binding of anti-SARS-CoV-2 RBD IgY to recombinant S1 full length (FL) of the index virus, the RBD of the Alpha and Beta variants, and the immunizing RBD of the index virus by ELISA. (D) Binding of anti-SARS-CoV-2 RBD IgY to the index virus and Omicron variant (B.1.1.529) RBD domain using ELISA. (E) Neutralization of pseudovirus (VSV-S) SARS-CoV-2 carrying S protein of index pseudovirus, Alpha, or Beta variants by anti-SARS-CoV-2 RBD IgY. (F) Neutralization of pseudoviruses listed in (E) by Human anti-SARS-CoV-2 RBD. (G) Neutralization of live index or Delta viruses by anti-SARS-CoV-2 IgY against the RBD. (H) Neutralization of live D614G vs. Delta variants by human serum of immunized individual or by anti-SARS CoV-2 IgY. Microscopic evaluation of monolayers of Vero E6 cells after 96 hours infection with the indicated authentic (live) SARS-CoV-2 variant. Images from infected cells are shown after 4 days of infection with SARS-CoV-2 variants in the absence or presence of test items. Top three panels: Infection in the presence of MEX-BC2/2020 and bottom three panels: infection with the Delta variant each in the presence of vehicle alone, serum of a person immunized twice with the Moderna (mRNA-1273) vaccine or anti-SARS CoV-2 RBD IgY, as indicated, all at the indicated concentration (neutralization experiments in panels E-H were conducted by RetroVirox using pseudovirus or live virus, as indicated). Except when indicated, the studies were done over several months; therefore, the absolute titers in the ELISA and neutralization studies were not identical. However, each experiment included the same positive control; index RBD for ELISA and index virus for neutralization assays.](https://www.news-medical.net/images/news/ImageForNews_701359_16419625388942409.jpg)
Common variants of SARS-CoV-2 and anti-SARS-CoV-2 IgY interaction with them. (A) A scheme depicting locations of mutated amino acids in Alpha through Mu variants of SARS-CoV-2, focusing on the RBD domain only. Each color bar indicates the amino acid in the index virus that was mutated in the variant. (B) Spike protein of SARS-CoV-2 Alpha, Beta, Delta, and Omicron are shown from left to right. Molecular Operating Environment was used to create the figure [31]. The location of mutations in the structure of the S protein trimer of SARS-CoV-2 (PDB ID: 7A98) for 4 of the common variants are indicated in red and glycosylation sites are indicated in pink throughout the S protein. Blue ribbon indicates RBD (amino acids 328-533) and the orange ribbon indicates receptor binding motif (amino acids 437- 508). (C) Binding of anti-SARS-CoV-2 RBD IgY to recombinant S1 full length (FL) of the index virus, the RBD of the Alpha and Beta variants, and the immunizing RBD of the index virus by ELISA. (D) Binding of anti-SARS-CoV-2 RBD IgY to the index virus and Omicron variant (B.1.1.529) RBD domain using ELISA. (E) Neutralization of pseudovirus (VSV-S) SARS-CoV-2 carrying S protein of index pseudovirus, Alpha, or Beta variants by anti-SARS-CoV-2 RBD IgY. (F) Neutralization of pseudoviruses listed in (E) by Human anti-SARS-CoV-2 RBD. (G) Neutralization of live index or Delta viruses by anti-SARS-CoV-2 IgY against the RBD. (H) Neutralization of live D614G vs. Delta variants by human serum of immunized individual or by anti-SARS CoV-2 IgY. Microscopic evaluation of monolayers of Vero E6 cells after 96 hours infection with the indicated authentic (live) SARS-CoV-2 variant. Images from infected cells are shown after 4 days of infection with SARS-CoV-2 variants in the absence or presence of test items. Top three panels: Infection in the presence of MEX-BC2/2020 and bottom three panels: infection with the Delta variant each in the presence of vehicle alone, serum of a person immunized twice with the Moderna (mRNA-1273) vaccine or anti-SARS CoV-2 RBD IgY, as indicated, all at the indicated concentration (neutralization experiments in panels E-H were conducted by RetroVirox using pseudovirus or live virus, as indicated). Except when indicated, the studies were done over several months; therefore, the absolute titers in the ELISA and neutralization studies were not identical. However, each experiment included the same positive control; index RBD for ELISA and index virus for neutralization assays.
Conclusion
One of the advantages of hen-derived IgY antibodies is that it has a reduced risk of severe immune responses. This is because these antibodies do not bind with the Fc receptor and rheumatoid factor or activate the human complement cascade. This characteristic feature widens its applicability to a broad range of persons, including immunocompromised, elderly, and children.
However, scientists have not described what would happen if these egg-derived immunoglobulins, containing potentially antigenic residual chicken proteins, were given to those who are allergic to egg yolks.
The authors suggested that until vaccination or herd immunity is achieved, intranasal delivery of anti-SARS-CoV-2 IgY could provide passive immunization or short-term protection against COVID-19 infection. These antibodies were found to be effective against all the circulating SARS-CoV-2 variants.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Frumkin, L. et al. (2022) Egg-derived anti-SARS-CoV-2 immunoglobulin Y (IgY) with broad variant activity as intranasal prophylaxis against COVID-19: preclinical studies and randomized controlled phase 1 clinical trial, medRxiv, 2022.01.07.22268914; doi: https://doi.org/10.1101/2022.01.07.22268914, https://www.medrxiv.org/content/10.1101/2022.01.07.22268914v1
- Peer reviewed and published scientific report.
Frumkin, Lyn R., Michaela Lucas, Curtis L. Scribner, Nastassja Ortega-Heinly, Jayden Rogers, Gang Yin, Trevor J. Hallam, et al. 2022. “Egg-Derived Anti-SARS-CoV-2 Immunoglobulin Y (IgY) with Broad Variant Activity as Intranasal Prophylaxis against COVID-19.” Frontiers in Immunology 13: 899617. https://doi.org/10.3389/fimmu.2022.899617. https://www.frontiersin.org/articles/10.3389/fimmu.2022.899617.