2. What should I know before I use STELARA?
Do not use if you have ever had an allergic reaction to ustekinumab or any of the
ingredients listed at the end of the CMI.
Talk to your doctor if you have any other medical conditions, take any other medicines,
or are pregnant or plan to become pregnant or are breastfeeding, or have an allergy
to latex.
3. What if I am taking other medicines?
Some medicines may interfere with STELARA and affect how it works.
4. How do I use STELARA?
STELARA is injected under the skin (subcutaneous)
5. What should I know while using STELARA?
|
Things you should do
|
Remind any doctor, dentist or pharmacist you visit that you are using STELARA.
Keep all your appointments.
Tell your doctor if you become pregnant and if you or your baby is scheduled for any
vaccines
|
|
Things you should not do
|
Do not stop using this medicine suddenly unless your doctor tells you to.
Do not receive a live vaccine while using STELARA
|
|
Driving or using machines
|
Be careful before you drive or use any machines until you know how STELARA affects
you. The effects on your ability to drive or use machines whilst taking STELARA are
not known.
|
|
Drinking alcohol
|
There is no information on the effects of taking STELARA with alcohol
|
|
Looking after your medicine
|
Store STELARA in the refrigerator (2 °C to 8 °C). Do not freeze. Keep vials or syringes
in the carton to protect your medicine from light. Do not shake.
|
6. Are there any side effects?
Side effects that require urgent medical attention include: Signs of an allergic reaction,
swollen face, lips, mouth or throat, cough, shortness of breath and fever.
This medicine is subject to additional monitoring. This will allow quick identification
of new safety information. You can help by reporting any side effects you may get.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems .
Active ingredient(s):
ustekinumab (rmc)
Full Consumer Medicine Information (CMI)
This leaflet provides important information about using STELARA. You should also speak to your doctor or pharmacist if you would like further information
or if you have any concerns or questions about using STELARA.
Where to find information in this leaflet:
1. Why am I using STELARA?
STELARA contains the active ingredient ustekinumab. Ustekinumab is a monoclonal antibody. Monoclonal antibodies are proteins that recognise
and bind to other unique proteins.
Ustekinumab blocks the action of two proteins in your body called interleukin 12 (IL-12)
and interleukin 23 (IL-23). IL-12 and IL-23 are made by your body's immune system.
In people with psoriasis, psoriatic arthritis, Crohn's disease or ulcerative colitis,
IL-12 and IL-23 can cause their immune system to attack normal healthy parts of their
body. Ustekinumab can block IL-12 and IL-23 from causing the immune system to attack.
STELARA
is used in adults and paediatric patients (children and adolescents) 6 years and older
with moderate to severe plaque psoriasis that is chronic (doesn't go away), adults
with active psoriatic arthritis (an inflammatory disease of the joints that is usually
accompanied by psoriasis), moderately to severely active Crohn's disease (an inflammatory
disease of the bowel) and moderate to severe ulcerative colitis (an inflammatory disease
of the bowel).
2. What should I know before I use STELARA?
Warnings
Do not use STELARA if:
you are allergic to ustekinumab, or any of the ingredients listed at the end of this
leaflet.
Always check the ingredients to make sure you can use this medicine.
you have an active infection
Check with your doctor if you:
have any kind of infection, even if it is very minor
have an infection that won't go away or a history of infection that keeps coming back
have had TB (tuberculosis), or if you have recently been near anyone who might have
TB
have or have had any type of cancer
have any new or changing lesions within psoriasis areas or on normal skin
have recently received or are scheduled to receive a vaccine. Patients receiving STELARA
should not receive live vaccines. Tell your doctor if anyone in your house needs a
vaccine. The viruses in some vaccines can spread to people with a weakened immune
system, and can cause serious problems
are receiving allergy immunotherapy.
have an allergy to latex. The needle cover on the pre-filled syringe and the needle
cover in the bottom cap of the pre-filled pen contain dry natural rubber (a form of
latex). This may cause allergic reactions in people who are sensitive to latex.
During treatment, you may be at risk of developing certain side effects. It is important
you understand these risks and how to monitor for them. See additional information
under Section
6. Are there any side effects?
Pregnancy and breastfeeding
Check with your doctor if you are pregnant or intend to become pregnant. Ask your
doctor for advice before taking this medicine.
Talk to your doctor if you are breastfeeding or intend to breastfeed.
STELARA may be used during a pregnancy if needed. STELARA may pass into your breastmilk
in very small amounts. Women who are breastfeeding should talk to their doctor about
whether or not to use STELARA as it might be excreted in breast milk.
3. What if I am taking other medicines?
Tell your doctor or pharmacist if you are taking any other medicines, including any
medicines, vitamins or supplements that you buy without a prescription from your pharmacy,
supermarket or health food shop.
Check with your doctor or pharmacist if you are not sure about what medicines, vitamins
or supplements you are taking and if these affect STELARA.
You should not receive a live vaccine while taking STELARA. If you used STELARA while
pregnant, tell you baby's doctor about your STELARA use before the baby receives any
vaccines, including live vaccines, such as the BCG vaccines (used to prevent tuberculosis),
rotavirus vaccine or any other live vaccines.
4. How do I use STELARA?
How much to take / use
For the treatment of psoriasis or psoriatic arthritis STELARA is given by injection
just under the skin (subcutaneously).
For the treatment of Crohn's Disease and ulcerative colitis, the first dose of STELARA
will be given by an intravenous infusion, which means that the medicine will be given
to you through a needle placed in a vein. After this starting dose, all future doses
of STELARA are given by injection just under the skin (subcutaneously).
All STELARA injections are for single use in one patient only.
STELARA is intended for use under the guidance and supervision of your doctor. In
children and adolescents 6 years and older with psoriasis, it is recommended that
STELARA be administered by a health care provider.
When you start treatment, STELARA may be injected by your healthcare provider. If
your doctor determines that it is appropriate, you or your caregiver may be able to
administer it under the skin yourself if you wish, after proper training in injection
technique. (See How much to use and Injecting STELARA under the skin yourself).
It is important to remember that the first dose of STELARA for the treatment of Crohn's
Disease or ulcerative colitis (intravenous infusion) will always be administered by
your healthcare provider.
How much to use
Your doctor will determine the correct dose of STELARA for you and how often you should
receive it. Make sure to discuss with your doctor when you will receive injections
and to come in for all your scheduled follow-up appointments.
For children and adolescents aged 6 years or older with psoriasis:
The doctor will work out the right dose for you, including the amount (volume) of
STELARA to be injected to give the right dose. The right dose for you will depend
on your body weight at the time each dose is given. If you weigh less than 60 kg,
the recommended dose is 0.75 mg of STELARA per kg body weight.
If you weigh 60 kg to 100 kg, the recommended dose is 45 mg STELARA.
If you weigh more than 100 kg, the recommended dose is 90 mg STELARA.
After the starting dose, you will have the next dose 4 weeks later, and then every
12 weeks.
Follow the instructions provided and use STELARA until your doctor tells you to stop.
How to use STELARA
STELARA is injected under the skin (subcutaneous injection).
It can be injected by the patient, or by someone else, such as a family member, friend
or carer.
Your first STELARA injection may be administered by your healthcare provider. In children
and adolescents 6 years and older, it is recommended that all doses of STELARA be
administered by a healthcare provider. However, your healthcare provider may decide
that it is right for you or your caregiver to learn how to inject STELARA under the
skin (subcutaneously) yourself. If you would like to self-inject STELARA under your
skin, you or your caregiver must be trained by a healthcare professional to prepare
an injection and give it to yourself. If you have not been trained, please contact
your healthcare provider to schedule a training session. Call your healthcare provider
if you have any questions about giving yourself an injection.
STELARA is given alone and not mixed with other liquids for injection.
STELARA should not be used:
after the expiry date on the label
if the seal is broken
if the liquid is discoloured, cloudy or you can see other particulate matter floating
in it
if you know, or think that it may have been exposed to extreme temperatures (such
as being accidentally frozen or heated)
Do not shake STELARA at any time.
Prolonged vigorous shaking may damage the product. If STELARA has been shaken vigorously,
don't use it. STELARA is not to be mixed with other liquids for injection.
STELARA injection under the skin (subcutaneous) is available in vial, pre-filled syringe,
and pre-filled pen.
Please refer to the 'Instructions for Use' leaflet for the pre-filled pen. Instructions
for use for vial and pre-filled syringe are provided below.
How to inject STELARA from a vial
1. Check Vials and Assemble materials:
Take the vial(s) out of the refrigerator. Make sure that it is the right dose. If
your dose is 45 mg you will receive one 45 mg vial. If your dose is 90 mg, you will
receive two 45 mg vials. If you receive two 45 mg vials for a 90 mg dose, you will
need to give yourself two injections one right after the other. Check with your healthcare
provider.
Children weighing less than 60 kg require a dose lower than 45 mg. Make sure you know
the proper amount (volume) and type of syringe needed for dosing. If you don’t know
the amount or type of syringe needed, contact your healthcare provider for further
instructions.
Check Expiration Date
Open the box and remove the vial. Check the expiration date on the vial and the label
of the box. If the expiration date has passed, don't use it.
Check Solution in Vial
Make sure the vial is not damaged. Look at the solution or liquid in the vial to make
sure that it is not cloudy and not frozen.
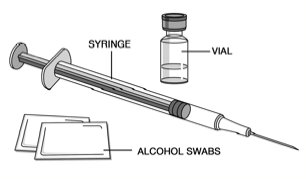
Assemble Additional Supplies
Assemble the additional supplies you will need for your injection. These include a
syringe with a 27 gauge, 1/2 inch needle, an alcohol swab, a cotton ball or gauze,
and a sharps container for syringe disposal.
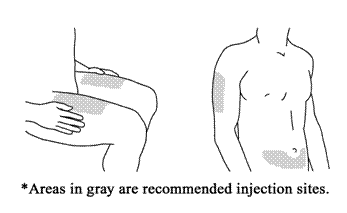
2. Choose the Injection Site:
Good sites are the top of the thigh and around the tummy (abdomen) but about 5 centimetres
away from the belly button (navel). Avoid, if possible, skin involved with psoriasis.
If your caregiver is giving you the injection, they may use the upper arms or buttocks
as well.
Prepare the Injection Site. Thoroughly wash your hands with soap and warm water. Wipe
the injection site with an alcohol swab. DO NOT touch this area again before giving
the injection.
3. Preparing the dose:
Remove the cap from the top of the vial but do not remove the stopper.
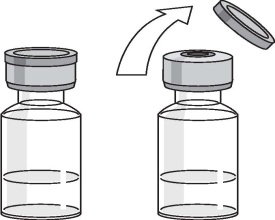
Clean the stopper with an antiseptic swab.
Remove the needle cover from the syringe. Do not touch the needle or allow the needle
to touch anything.
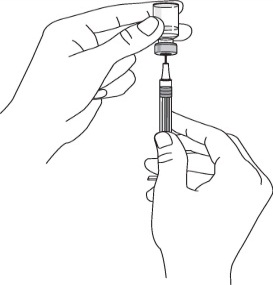
Put the vial on a flat surface and push the syringe needle through the rubber stopper.
Turn the vial and the syringe upside down.
For adults and paediatric patients (children and adolescents) 6 years of age and older,
who weigh 60 kg or more, pull on the syringe plunger to fill the syringe with the
amount of liquid prescribed by your healthcare provider (0.5 mL or 1.0 mL).
For children and adolescents 6 years of age or older who weigh less than 60 kg, the
amount of liquid prescribed by your healthcare provider may be less than 0.5 mL. Your
healthcare provider will recommend how much liquid is needed.
It is important that the needle is always in the liquid in order to prevent air bubbles
from forming in the syringe.
Remove the needle from the vial. Hold the syringe with the needle pointing up to see
if it has any air bubbles inside.
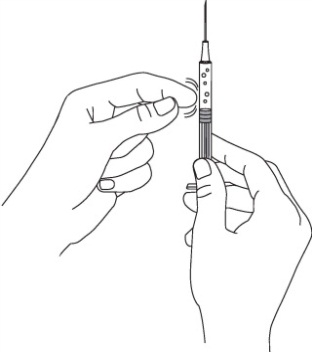
If there are air bubbles tap the side gently until the air bubbles go to the top of
the syringe and press the plunger until all of the air (but none of the liquid) has
been removed. Do not lay the syringe down or allow the needle to touch anything.
4. Injecting the Medication:
Gently pinch the cleaned skin between your thumb and index finger. Don't squeeze it.
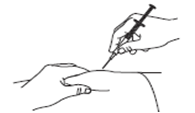
Push the syringe needle into the pinched skin.
Push the plunger with your thumb as far as it will go to inject all of the liquid.
Push it slowly and evenly, keeping the skin gently pinched.
When the plunger is pushed as far as it will go, take out the needle and let go of
the skin.
Press an antiseptic swab over the injection site for a few seconds after the injection.
Dispose of the Empty Syringe
Immediately dispose of the empty syringe into the sharps container. For your safety
and health and for the safety of others, needles and syringes must NEVER be re-used.
Dispose of sharps container according to your local regulations. See "After using
STELARA - Disposal".
Use a Cotton Ball or Gauze
There may be a small amount of blood or liquid at the injection site, which is normal.
You can press a cotton ball or gauze over the injection site and hold for 10 seconds.
Do not rub the injection site. You may cover the injection site with a small adhesive
bandage, if necessary.
If your dose amount is 90 mg, and you receive two 45 mg vials you may need to give
a second injection right after the first. Use a new needle and syringe. Choose a different
site for the second injection.
If you do not understand the instructions provided with this medicine, ask your doctor
or pharmacist for help.
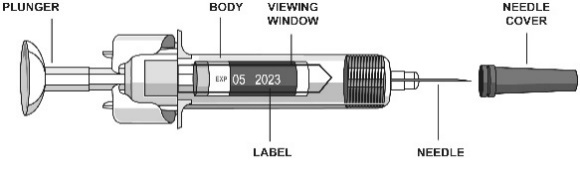
How to inject STELARA under the skin from a pre-filled syringe
To reduce the risk of accidental needle stick injuries to users, each pre-filled syringe
is equipped with a needle guard that is automatically activated to cover the needle
after complete delivery of the syringe content.
1. Preparing for pre-filled syringe use
Take the syringe(s) out of the refrigerator.
Make sure that it is the right dose.
Check Expiration Date
Open the box and remove the syringe. Check the expiration date on the pre-filled syringe
and the label of the box. If the expiration date has passed, don’t use it.
Check Solution in Syringe
Hold the pre-filled syringe with the covered needle pointing upward. Make sure the
syringe is not damaged. Look at the solution or liquid in the vial to make sure that
it is not cloudy and not frozen.
Assemble Additional Supplies
Assemble the additional supplies you will need for your injection. These include an
antiseptic wipe, a cotton ball or gauze, and a sharps container for syringe disposal.
DO NOT remove the needle cover from the pre-filled syringe.
DO NOT pull back on the plunger head at any time.
2. Choosing and preparing the injection site
Choose the Injection Site*
Good sites are the top of the thigh and around the tummy (abdomen) but about 5 centimetres
away from the belly button (navel). Avoid, if possible, skin involved with psoriasis.
If your caregiver is giving you the injection, they may use the upper arms or buttocks
as well.

Prepare the Injection Site
Thoroughly wash your hands with soap and warm water. Wipe the injection site with
an antiseptic wipe. DO NOT touch this area again before giving the injection.
3. Injecting the medication
Remove the Needle Cover
When you are ready to inject, pick up the pre-filled syringe with one hand and pull
the needle cover straight off.
Throw the needle cover into the rubbish.
You may notice a small air bubble in the pre-filled syringe. You do not need to remove
the air bubble. You may also see a drop of liquid at the end of the needle – this
is normal. Do not touch the needle or allow it to touch any surface.
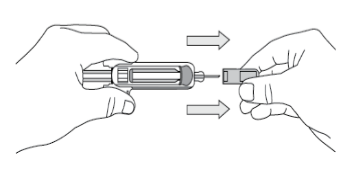
Note: The needle cover should NOT be removed until you are ready to inject the dose.
Do not use the syringe if it is dropped without the needle cover in place. If you
drop the syringe without the needle cover in place, please contact your healthcare
provider for assistance.
4. Inject the medication
Gently pinch the cleaned skin between your thumb and index finger.
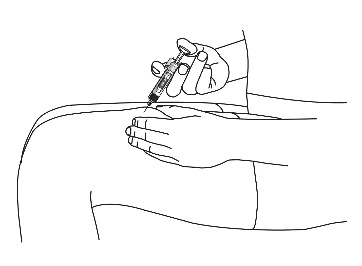
Don’t squeeze it. Push the syringe needle into the pinched skin.
Push the plunger with your thumb as far as it will go to inject all of the liquid.
Push it slowly and evenly, keeping the skin gently pinched.
When the plunger meets the end of the syringe barrel, and all of the medication has
been injected, release the pinched skin and gently remove the needle. Following complete
injection, the needle guard will automatically extend over the needle and lock as
you take your hand off the plunger.
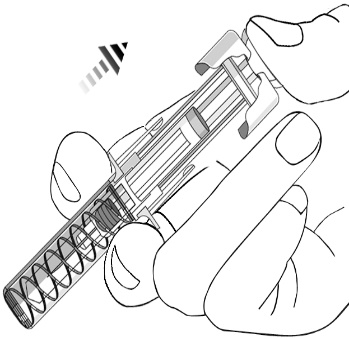
5. After the injection
Dispose of the Empty Syringe
Immediately dispose of the empty syringe into the sharps container. For your safety
and health and for the safety of others, needles and syringes must NEVER be re-used.
Dispose of sharps container according to your local regulations. See ‘After using
STELARA - Disposal’.
Use a Cotton Ball or Gauze
There may be a small amount of blood or liquid at the injection site, which is normal.
You can press a cotton ball or gauze over the injection site and hold for 10 seconds.
Do not rub the injection site. You may cover the injection site with a small adhesive
bandage, if necessary.
If you do not understand the instructions provided with this medicine, ask your doctor
or pharmacist for help.
Educational support
The Johnson & Johnson Immunology Patient Support Program is available to patients
prescribed STELARA. It offers:
Injection education
Ongoing medical supplies
Starter kit
Reminder service
Ongoing education
If you forget to use STELARA
Make the next injection as soon as you remember, and then continue to use it as you
would normally.
Do not administer a double dose to make up for the dose you missed.
If you have missed more than one dose, or are not sure what to do, check with your
doctor or pharmacist.
If you have trouble remembering when to use your medicine, ask your pharmacist for
some hints
If you use too much STELARA
If you think that you have used too much STELARA, you may need urgent medical attention.
You should immediately:
phone the Poisons Information Centre
(by calling
13 11 26), or
contact your doctor, or
go to the Emergency Department at your nearest hospital.
You should do this even if there are no signs of discomfort or poisoning.
5. What should I know while using STELARA?
Things you should do
Always follow your doctor's instructions carefully.
Tell your doctor about your medical conditions before each treatment (see Before you
use STELARA).
Tell your doctor if you become pregnant while using STELARA.
If you used STELARA while pregnant, tell you baby's doctor about your STELARA use
before the baby receives any vaccines, including live vaccines, such as the BCG vaccine
(used to prevent tuberculosis), rotavirus vaccine or any other live vaccines.
If you develop headache, vision problems, seizures or change in mental status (for
example confusion), tell your doctor immediately.
If you are about to start taking a new medicine, tell your doctor and pharmacist that
you are using STELARA.
Call your doctor straight away if you:
get symptoms of an infection, such as a fever, skin sores or feeling tired
become pregnant while using STELARA. Remind any doctor, dentist or pharmacist you
visit that you are using STELARA.
Things you should not do
Do not stop using this medicine suddenly without checking with your doctor
You should not receive a live vaccine while taking STELARA.
Do not use STELARA to treat any other complaint unless your doctor says so.
Do not give this medicine to anyone else, even if their symptoms seem similar to yours.
Driving or using machines
Be careful before you drive or use any machines or tools until you know how STELARA
affects you.
Drinking alcohol
Tell your doctor if you drink alcohol.
There is no information on the effects of taking STELARA with alcohol
Looking after your medicine
Follow the instructions in the carton on how to take care of your medicine properly.
Store STELARA between 2°C and 8°C in the refrigerator. Do not freeze.
Keep the product in the original carton to protect from light until the time of use.
Do not shake.
Do not store STELARA in the bathroom or near a sink, or in the car or on window sills.
Keep it where young children cannot reach it.
STELARA pre-filled syringes - room temperature storage (excluding vials)
If needed, you may store STELARA pre-filled syringes and pre-filled pens at room temperature
up to 30°C for one period of up to 30 days.
Write the date on the carton, when removed from the refrigerator.
Once pre-filled syringe or pre-filled pen has been stored at room temperature, do
not put it back in the refrigerator.
Throw away pre-filled syringe or pre-filled pen if it has been kept for 30 days and
has not been used or reached its original expiry.
For pre-filled pen only, before use, remove the carton from the refrigerator and keep
the pre-filled pen inside the carton and allow to reach room temperature by waiting
for 30 minutes.
Getting rid of any unwanted medicine
After injection, used syringes should be placed in a puncture-resistant container,
like a sharps container. Dispose of your sharps container according to your state
or local regulations. Empty vials, antiseptic wipes, and other supplies can be placed
in regular rubbish.
If your doctor tells you to stop using STELARA, or your medicine has passed its expiry
date, ask your pharmacist what to do with any medicine that may be left over.
6. Are there any side effects?
All medicines can have side effects. If you do experience any side effects, most of
them are minor and temporary. However, some side effects may need medical attention.
See the information below and, if you need to, ask your doctor or pharmacist if you
have any further questions about side effects.
Tell your doctor if you notice any other side effects, particularly if they bother
you or do not go away. In general, the side effects of STELARA in children and adolescents
6 years and older are similar to those in adults. Ask your doctor or pharmacist for
more information.
Less serious side effects
Serious side effects
Tell your doctor or pharmacist if you notice anything else that may be making you
feel unwell.
Other side effects not listed here may occur in some people. Tell your doctor if you
notice any other side effects, particularly if they bother you or do not go away.
In general, the side effects of STELARA in children and adolescents 6 years and older
are similar to those in adults. Ask your doctor or pharmacist for more information.
Reporting side effects
After you have received medical advice for any side effects you experience, you can
report side effects to the Therapeutic Goods Administration online at
www.tga.gov.au/reporting-problems . By reporting side effects, you can help provide more information on the safety of
this medicine.
Always make sure you speak to your doctor or pharmacist before you decide to stop
taking any of your medicines.
7. Product details
This medicine is only available with a doctor's prescription.
What STELARA contains
|
Active ingredient
(main ingredient)
|
ustekinumab
|
|
Other ingredients
(inactive ingredients)
45 mg and 90 mg
|
histidine/histidine hydrochloride monohydrate
sucrose
polysorbate 80
water for injections.
|
|
Other ingredients
(inactive ingredients)
130 mg
|
Histidine
Histidine hydrochloride monohydrate
Sucrose
Polysorbate 80
Methionine
Disodium edetate
Water for injections
|
No preservatives are present.
Do not take this medicine if you are allergic to any of these ingredients.
What STELARA looks like
STELARA is injection for subcutaneous use is a colourless to light yellow solution
and may contain a few small translucent or white particles of protein. This appearance
is not unusual for solutions containing protein.
STELARA solution for intravenous use is a clear, colourless to light yellow product.
STELARA is available in the following presentations:
For subcutaneous administration
STELARA vial: 45 mg ustekinumab in 0.5 mL (for subcutaneous use) (AUST R 149549)
STELARA pre-filled syringe: 45 mg ustekinumab in 0.5 mL (for subcutaneous use) (AUST
R 165953)
STELARA pre-filled syringe: 90 mg ustekinumab in 1.0 mL (for subcutaneous use) (AUST
R 165954)
STELARA ustekinumab 45 mg/0.5 mL solution for injection pre-filled pen (One-Press®
patient-controlled injector) (for adult use) (AUST R 400550)
STELARA ustekinumab 90 mg/1 mL solution for injection pre-filled pen (One-Press® patient-controlled
injector) (for adult use) (AUST R400551)
For intravenous infusion
STELARA vial:130 mg in 26 mL (concentrate for intravenous infusion only) (AUST R 282906)
Each box contains 1 unit.
Who distributes STELARA
Janssen-Cilag Pty Ltd
17 Khartoum Road,
Macquarie Park,
NSW 2113,
Australia
Telephone: 1800 226 334
NZ Office: Auckland New Zealand
Telephone: 0800 800 806
This leaflet was prepared in January 2026.