This article and associated images are based on a poster originally authored by Hannah Sharplin, Stuart Prime, Jessica Tilman, Steven Broadbent, David Wallbank and Ashley Barnes and presented at ELRIG Drug Discovery 2025 in affiliation with Axol Bioscience Ltd.
This poster is being hosted on this website in its raw form, without modifications. It has not undergone peer review but has been reviewed to meet AZoNetwork's editorial quality standards. The information contained is for informational purposes only and should not be considered validated by independent peer assessment.

Abstract
Amyotrophic Lateral Sclerosis (ALS) is a progressively fatal neurodegenerative disease that damages nerve cells in the central nervous system (CNS), but its effects are manifested in the peripheral nervous system (PNS) due to the loss of motor neuron function, resulting in muscle weakness and atrophy. Although -85-90 % of ALS cases are sporadic, mutations in over 40 genes have been identified that contribute to the development of ALS (1).
There is no cure for ALS and approved treatments are primarily aimed at addressing symptoms. Lack of standardized, easy-to-use, consistent and readily accessible human cell models has been a hurdle in early- stage ALS drug discovery. Human induced pluripotent stem cell (iPSC)-derived disease models offer the promise of building better in-vitro models for increased translational relevance.
Hyperexcitability is a trait in ALS neuronal pathology, contributing to the degeneration of motor neurons and disease progression. As such, this should be an essential characteristic of any physiologically-relevant ALS disease model.
Here, we demonstrate the successful characterization of axoCellsTM human iPSC-derived motor neurons from six different donor lines including two unaffected donors, one C9orf72 carrying donor (the sibling to the C9orf72 ALS donor), and three donors each carrying significant genotypes associated with ALS; SOD1, C9orf72 and TDP43 for ALS drug discovery.
Using functional screening, the cells were shown to demonstrate a disease relevant phenotype when compared to the control. Through Brightfield imaging and Immunocytochemistry (ICC), the morphology and expression of neuronal marker TUJ-1 (Neuron-specific class III beta-tubulin) was observed.
Spontaneous Neuronal activity (SNA) and Multi-electrode array (MEA) analysis displayed overall firing rates, synchronization of firing, length of bursts and amplitude of bursts consistent with the cells' phenotype. Specifically, the ALS donor cells displayed a reproducible loss of synchronous firing and different degrees of hyperexcitability.
Multiple manufacturing runs showed batch-to-batch functional consistency with these results. Such reproducible functional characterization allows the integration of functional testing into the standard QC workflow thus, demonstrating improved consistency and product quality. Successful characterization of these human iPSC-derived cells which display attributes in line with ALS clinical pathology, confirms their suitability to function as a physiologically-relevant disease model for ALS drug discovery.
Methods
Frozen motor neuron progenitors from unaffected donor cells were assay-ready from as early as 10 days post-thaw using our motor neuron Accelerator, while SOD1 and TDP43 phenotypes required 20 days post- thaw to mature (Fig. 1). Brightfield imaging was performed at 10 days in vitro (DIV10) to characterize neuronal cell morphology and the formation of structural neuronal networks.
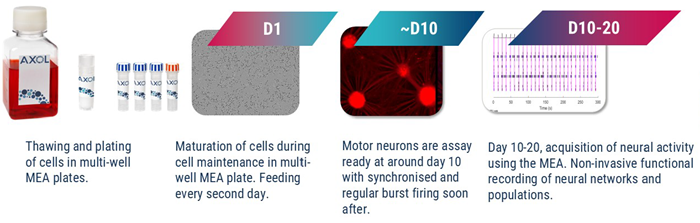
Figure 1. Schematic of protocol from thawing of motor neuron precursors to MEA measurements. Image Credit: Image courtesy of Hannah Sharplin et al., in partnership with ELRIG (UK) Ltd.
Further imaging through ICC was used to evaluate the expression of neuronal cell marker TUJ-1, facilitating the identification of neuronal differentiation and maturation. To measure neuronal activity development, we used the Axion Biosystems Maestro Pro MEA platform, which allowed for real-time recording of electrical activity from neuronal networks. Motor neuron activity was measured and the synchronicity of this activity was analyzed using the Sartorius IncuCyte S3 system to confirm the functionality of the neurons.
Results
Characterization by cell morphology
The unaffected donor cells displayed uniform cultures with large cell bundles and thick cabling while the C9orf72 carrying donor cells (ax0073) displayed smaller irregular cell-body clusters that are dispersed with thinner cabling between cell bundles. The SOD1 and TDP43 donor cells displayed more heterogenous morphologies each with varied cluster sizes and neurite characteristics. The C9orf72 donor cells (ax0074) displayed fewer uniform cultures, with smaller bundles and fibrous neurites.
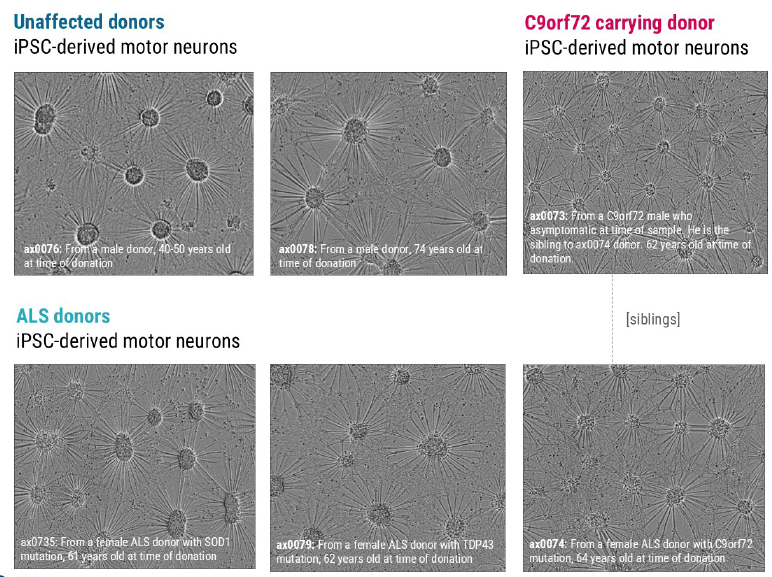
Figure 2. Brightfield Imaging at 10 days in vitro (DIV10) showed the expected neuronal morphology of neurite outgrowth and the formation of structural neuronal networks. Image Credit: Image courtesy of Hannah Sharplin et al., in partnership with ELRIG (UK) Ltd.
Expression of neuronal cell marker TUJ-1 via Immunocytochemistry
Following ICC imaging, cells demonstrated expression of the neuronal microtubule marker TUJ-1 and an identifiable morphology consistent with their phenotype in line with Figure 2. Both methods also displayed clear phenotypic morphological differences between the ALS donor lines.

Figure 3. ICC imaging at 10 days in vitro (DIV10) demonstrate microtubule marker expression (TUJ-1) of axoCells Motor Neurons. Image Credit: Image courtesy of Hannah Sharplin et al., in partnership with ELRIG (UK) Ltd.
Spontaneous Neuronal Activity measurement using the Sartorius IncuCyte® S3
Distinct patterns of SNA among the donor cells were observed. The unaffected donor cells exhibited highly synchronized firing, characterized by a higher mean burst duration and a lower burst rate. In contrast, the C9orf72 affected donor cells displayed a hyperexcitable phenotype, showing less synchronized firing. Notably, all affected donor cells (C9orf72, SOD1, TDP43) demonstrated an increased burst rate, indicating an increase in neuronal excitability typical of hyperexcitability. The C9orf72 and TDP43 donor cells also displayed less synchronized firing.

Image Credit: Image courtesy of Hannah Sharplin et al., in partnership with ELRIG (UK) Ltd.
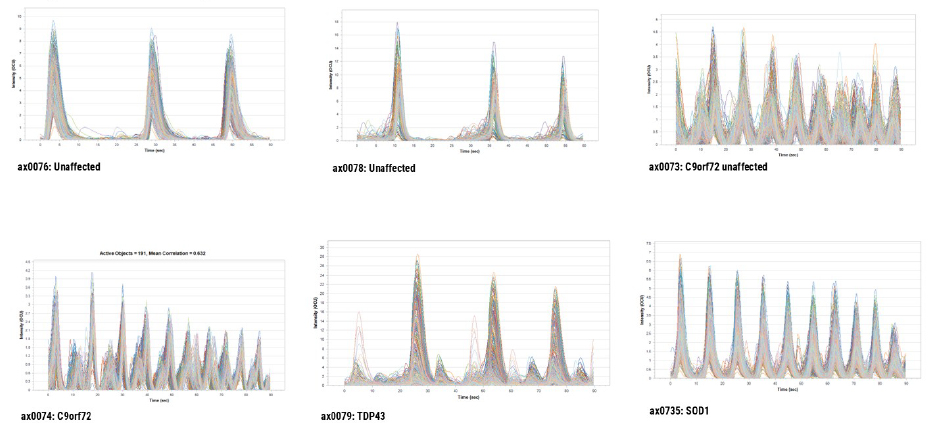
Figure 4. Analysis from recording of spontaneous neuronal activity measured on the Sartorius IncuCyte® S3. IncuCyte® Neuroburst Orange lentiviral reagent was used to image spontaneous neuronal firing in iPSC-derived motor neurons from unaffected donors and ALS donors. Image Credit: Image courtesy of Hannah Sharplin et al., in partnership with ELRIG (UK) Ltd.
Motor neuron activity measurement using Multi-electrode Array
Data observed through MEA detection were comparable to SNA data. MEA data confirmed that ALS motor neurons displayed less synchronized firing and a more hyperexcitable phenotype compared to the unaffected donor cells.

Image Credit: Image courtesy of Hannah Sharplin et al., in partnership with ELRIG (UK) Ltd.
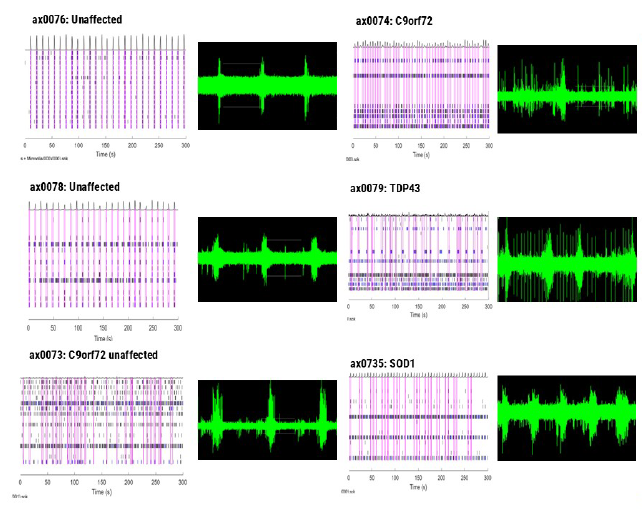
Figure 5. Measurement of motor neuron activity of unaffected and ALS donor cells using MEA. Image Credit: Image courtesy of Hannah Sharplin et al., in partnership with ELRIG (UK) Ltd.
Table 1. Changes in the parameter are indicated by color and arrow direction, as described in the key on the right. Source: ELRIG (UK) Ltd.
| Firing parameter |
Wildtype |
C9orf72 |
SOD1 |
TDP43 |
| No. of spikes |
 |
 |
 |
 |
| Mean firing rate |
 |
 |
 |
 |
| No. of spikes per burst |
 |
 |
 |
 |
| Inter-burst interval |
 |
 |
 |
 |
| ISI-COV |
 |
 |
 |
 |
| No. of bursts |
 |
 |
 |
 |
| Burst duration |
 |
 |
 |
 |
| No. of network bursts |
 |
 |
 |
 |
| Network burst duration |
 |
 |
 |
 |
| No. of spikes per NB |
 |
 |
 |
 |
| Network burst % |
 |
 |
 |
 |
| Network normalised duration IQR |
 |
 |
 |
 |
| Synchrony index |
 |
 |
 |
 |
| Spike amplitude |
 |
 |
 |
 |
| Table Key |
|
|
| Increased parameter value (major/small) |
 |
 |
| Decreased parameter value (major/small) |
 |
 |
| Comparable to wildtype |
 |
Conclusion
In the characterization of this set of axoCells iPSC-derived motor neurons, we used MEA and imaging to observe key traits. By incorporating this characterization into manufacturing QC and functional QC processes, we recorded multiple lines and multiple production runs (differentiations) to ensure these traits were robust and reproducible and were not just a consequence of a single differentiation procedure.
Motor neurons from 6 different lines were assessed for ALS-related morphology and neural activity phenotypes. The unaffected control lines exhibited consistent spike amplitude, short burst events, and synchrony. In comparison, motor neurons from the ALS lines demonstrated a reproducible loss-or gain-of- function for certain parameters in each phenotype with the same patterns observed across multiple platforms. ALS motor neurons with different mutations displayed distinct phenotypes from each other highlighting variations that exist amongst patients with varying mutations. This showcases the opportunity for patient stratification in ALS drug discovery and development.
In addition, such reproducible functional characterization of these cells allows for functional QC to be incorporated into the standard QC testing of iPSC-derived motor neurons improving the QC process and demonstrating batch-to-batch consistency.
All ALS phenotype lines displayed a reproducible loss of synchronous firing and different degrees of hyperexcitability. This is in accordance with expected ALS clinical pathology and supports the case for the use of these cells in experimental in vitro models to study ALS pathology and potential therapeutics.
References
Gregory, J.M., et al. (2020). Genetics of Amyotrophic Lateral Sclerosis. Current Genetic Medicine Reports, 8(4), pp.121–131. https://doi.org/10.1007/s40142-020-00194-8.
About AXOL Biosciences
Axol specializes in human cell culture.
Axol produces high quality human cell products and critical reagents such as media and growth supplements. We have a passion for great science, delivering epic support and innovating future products to help our customers advance faster in their research.
Our expertise includes reprogramming cells to iPSCs and then differentiating to various cell types. We supply differentiated cells derived from healthy donors and patients of specific disease backgrounds. As a service, we also take cells provided by customers (primary or iPSC) and then do the reprogramming (when necessary) and differentiation. Clearly, by offloading the burden of generating cells, your time is freed up to focus on the research. Axol holds the necessary licenses that are required to do iPSC work.
About ELRIG (UK) Ltd.
The European Laboratory Research & Innovation Group (ELRIG) is a leading European not-for-profit organization that exists to provide outstanding scientific content to the life science community. The foundation of the organization is based on the use and application of automation, robotics and instrumentation in life science laboratories, but over time, we have evolved to respond to the needs of biopharma by developing scientific programmes that focus on cutting-edge research areas that have the potential to revolutionize drug discovery.
Comprised of a global community of over 12,000 life science professionals, participating in our events, whether it be at one of our scientific conferences or one of our networking meetings, will enable any of our community to exchange information, within disciplines and across academic and biopharmaceutical organizations, on an open access basis, as all our events are free to attend!
Our values
Our values are to always ensure the highest quality of content, that content will be made readily accessible to all, and that we will always be an inclusive organization, serving a diverse scientific network. In addition, ELRIG will always be a volunteer-led organization, run by and for the life sciences community, on a not-for-profit basis.
Our purpose
ELRIG is a company whose purpose is to bring the life science and drug discovery communities together to learn, share, connect, innovate and collaborate, on an open access basis. We achieve this through the provision of world-class conferences, networking events, webinars, and digital content.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last Updated: Nov 27, 2025