This article and associated images are based on a poster originally authored by O Manners, G Pergl-Wilson, T Khanam, H Weisser, T Samuels, H Fischl, J Thottachery, M Pawlowska, S Ashraf, B Andrews, H Harrison, E Anderson, and O Rausch, and presented at ELRIG Drug Discovery 2025 in affiliation with Storm Therapeutics Limited and Nikon Bioimaging Lab Europe.
This poster is being hosted on this website in its raw form, without modifications. It has not undergone peer review but has been reviewed to meet AZoNetwork's editorial quality standards. The information contained is for informational purposes only and should not be considered validated by independent peer assessment.

Introduction
Alternative lengthening of telomeres (ALT) is a telomere maintenance mechanism employed by 5-10 % of cancer cells to achieve replicative immortality, and is absent in normal cells. Unlike telomerase (TEL) positive cancers, ALT tumors rely on chronic telomeric replication stress to drive telomere extension.
Factors regulating this process serve as promising targets for the development of specific therapeutic inhibitors for oncology. Despite this, no targeted therapies currently exist for ALT cancers. Through collaboration with the Nikon Bioimaging lab, the research team performed a high-throughput arrayed CRISPR screen with an imaging output to identify RNA-interacting factors with potential as targets in ALT cancers.
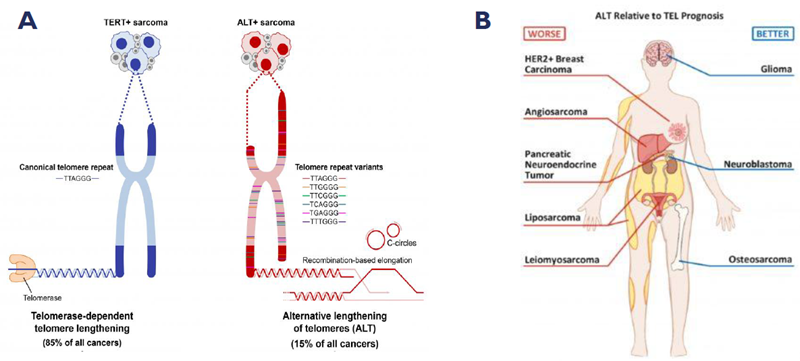
A. Tumors use two predominant mechanisms to maintain telomere length, ALT and TEL. Image Credit: Nucleic acids research, Frank et al 2012; B. ALT is associated with poor prognosis in a range of different cancer types. Image Credit: MDPI cancers, Mackenzie Jr et al 2021
In-house assay development to measure ALT activity
To discover and validate therapeutic targets for ALT cancers, the team adapted and implemented two assays from published literature, which can detect unique ALT molecular hallmarks: CCA-qPCR for C-circles and ssTelo native FISH for single-stranded telomeric DNA.
By performing CRISPR knockout (KO) of literature-established control genes, they validated these assays by observing the expected increase (FANCM) or decrease (BLM) in ALT hallmarks associated with loss of either gene.
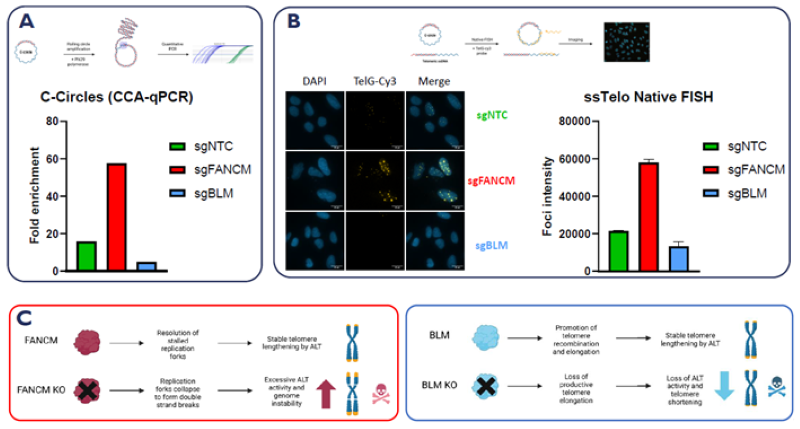
A. CCA-qPCR assay. C-circles are amplified from genomic DNA by rolling circle amplification, and fold change is detected using telomere-specific primers. B. Representative images from the Native-FISH assay where telomeric ssDNA is detected as nuclear foci by a TelG-Cy3 probe in U-2OS cells. Quantification of TelG-Cy3 foci intensity in U-2OS cells treated with control sgRNAs. C. Schematic depicting the function of established ALT control genes FANCM and BLM. Image Credit: Image courtesy of Manners O et al., in partnership with ELRIG (UK) Ltd.
Generation of a cross-lineage ALT/TEL cell line panel
Osteosarcoma models feature heavily in the ALT literature due to the high prevalence of ALT in clinical samples from this lineage and the availability and amenability of these cell lines to in vitro manipulation. The team used the CCA-qPCR and ssTelo native FISH assays to validate a panel of osteosarcoma cell lines for ALT or TEL status based on the presence or absence of the ALT hallmarks.
They also identified ALT cell lines from brain, soft tissue, and lung to expand their ability to identify ALT-specific targets across multiple lineages.
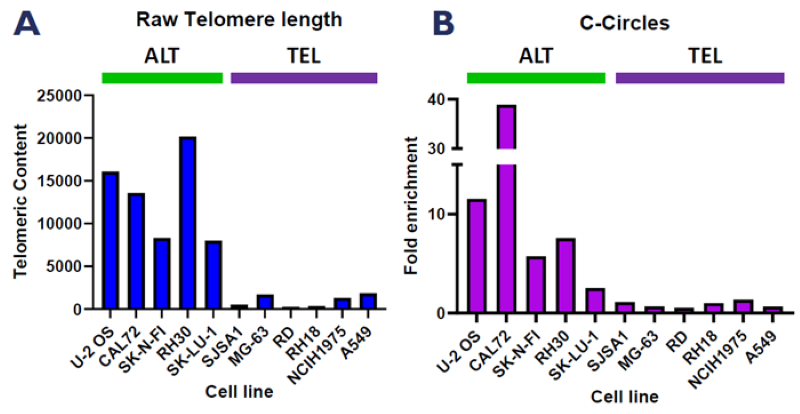
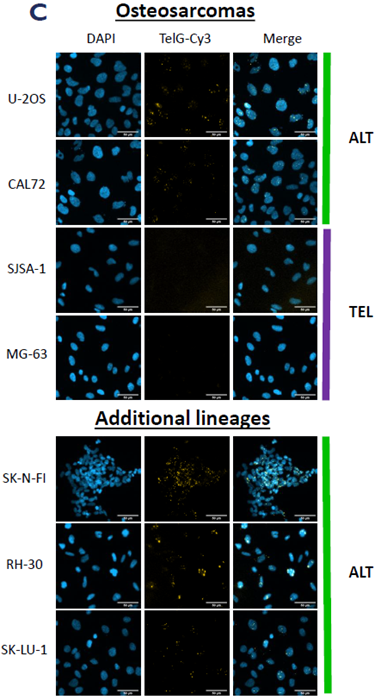
A. qPCR measurement of raw telomere length in an ALT/TEL cell line panel using a telomere-specific primer set. B. CCA-qPCR data highlighting fold enrichment in telomeric DNA after RCA of C-circles. C. N-FISH images detecting ALT activity in a panel of ALT and TEL cell lines. Image Credit: Image courtesy of Manners O et al., in partnership with ELRIG (UK) Ltd.
A high-throughput arrayed KO imaging screen identifies RNA interactors regulating ALT biology
To identify ALT modulators within STORM’s target space, the team carried out an arrayed KO screen in the ALT-positive osteosarcoma cell line U-2OS and, utilizing the imaging and analysis expertise of the Nikon Bioimaging Lab, determined the effects of these KOs on ssTelo-positive foci number and intensity.
The results of their screen confirmed several known ALT modulators: co-factors of FANCM and BLM, FAAP24 and RMI1, the dead-box helicases DDX39A and DDX39B, and members of the cleavage and polyadenylation machinery, CSPF2 and PABPN1. They also identified several novel regulators of ALT activity that could be pursued as potential therapeutic targets for ALT-related cancers.
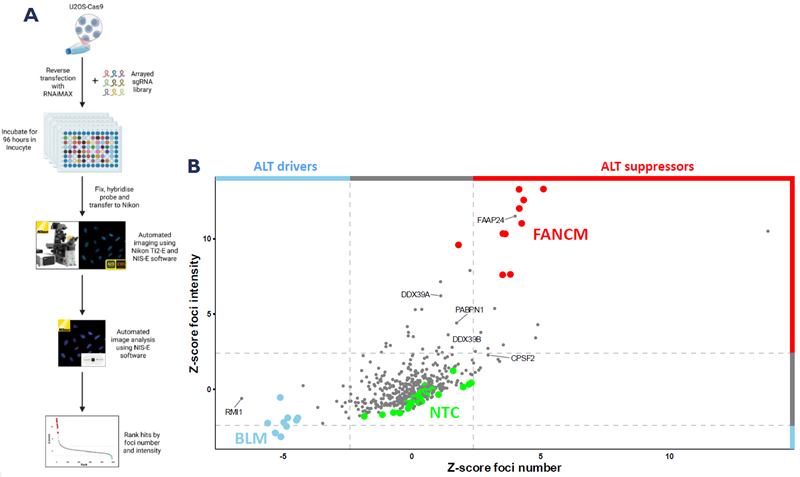
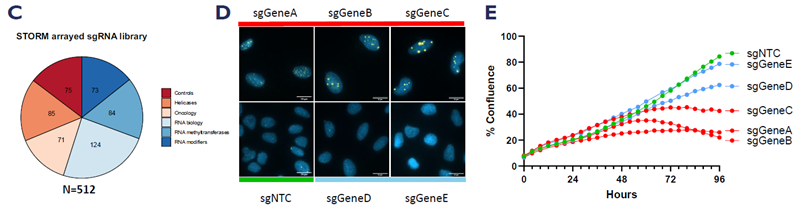
A. Schematic representation of the workflow used to identify modulators of ALT activity. B. Scatterplot showing quantification of foci intensity and number for 512 gene knockouts. C. Composition of the STORM sgRNA library. D. Representative images of ALT suppressors and drivers identified by the screen. E. Incucyte growth curves showing growth defect for loss of genes in Figure D. Image Credit: Image courtesy of Manners O et al., in partnership with ELRIG (UK) Ltd.
Validation screen verifies on target editing and confirms hits from the primary screen
The team performed a confirmatory screen, focusing on the hits from the first screen along with any associated complex members, regardless of their prior performance. They used ICE analysis to demonstrate very high on-target editing efficiency, indicating a low likelihood of false negatives within the primary screen.
They observed a strong correlation in the ssTelo FISH results between the primary and secondary screens, confirming the ALT modulatory activity of the top hits.
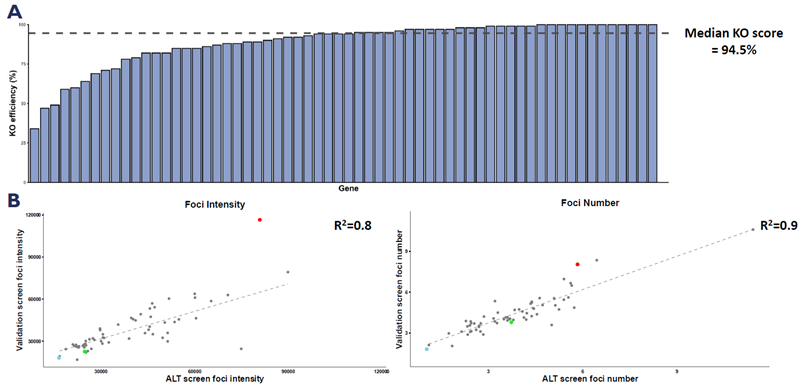
A. KO scores determined by Inference of CRISPR edits (ICE) software for the entire panel of genes selected for the follow-up validation screen. B. Scatterplots highlighting correlation between ALT and validation screens for foci number and intensity. Image Credit: Image courtesy of Manners O et al., in partnership with ELRIG (UK) Ltd.
Summary
- Through collaboration with the Nikon Bioimaging lab, STORM has run a high-throughput arrayed KO screen to identify RNA-interacting factors with potential as therapeutic targets for ALT cancers.
- A total of 54 putative ALT modulators were identified with Z score <-2.4 or >2.4. Some of these hits have been described previously, but many are novel regulators of ALT biology.
- Hits from the initial ALT screen show a strong correlation with a follow-up validation screen.
Next steps
- Top hits from their screens will be depleted in a multi-lineage ALT/TEL cell panel to identify ALT-specific dependencies with potential as therapeutic targets.
About STORM Therapeutics 
STORM Therapeutics is a clinical-stage biotechnology company pioneering cellular reprogramming through RNA modifications to treat disease.
Its world leading understanding of RNA modifying enzymes (RME) has led to the discovery of breakthrough small molecule drugs that precisely reprogram cells through RNA biology for the treatment of cancer, inflammation, viruses and CNS diseases.
About ELRIG (UK) Ltd.
The European Laboratory Research & Innovation Group (ELRIG) is a leading European not-for-profit organization that exists to provide outstanding scientific content to the life science community. The foundation of the organization is based on the use and application of automation, robotics and instrumentation in life science laboratories, but over time, we have evolved to respond to the needs of biopharma by developing scientific programmes that focus on cutting-edge research areas that have the potential to revolutionize drug discovery.
Comprised of a global community of over 12,000 life science professionals, participating in our events, whether it be at one of our scientific conferences or one of our networking meetings, will enable any of our community to exchange information, within disciplines and across academic and biopharmaceutical organizations, on an open access basis, as all our events are free-of-charge to attend!
Our values
Our values are to always ensure the highest quality of content and that content will be made readily accessible to all, and that we will always be an inclusive organization, serving a diverse scientific network. In addition, ELRIG will always be a volunteer led organization, run by and for the life sciences community, on a not-for-profit basis.
Our purpose
ELRIG is a company whose purpose is to bring the life science and drug discovery communities together to learn, share, connect, innovate and collaborate, on an open access basis. We achieve this through the provision of world class conferences, networking events, webinars and digital content.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last Updated: Nov 13, 2025