This article is based on a poster originally authored by Gareth Berry, Amy Hawksworth, Damon Ho, Ben Johnson, Nick Pearman, and Beata Adamiak.
Introduction
Pharmaron Biologics, Liverpool, UK, is a commercial development and manufacturing organization (CDMO) that offers a range of unique end-to-end laboratory services for the development and manufacture of complex biological medicines, including Gene Therapies.
Pharmaron has successfully developed a platform procedure that can be customized for a client's particular AAV gene therapy product.
Using fast manufacturing feasibility assessments, high-throughput process development methodologies, and Pharmaron’s proprietary tiered-testing approach, Pharmaron can move from initial assessment to GMP-ready status in as little as six months.
Pharmaron’s process development capabilities
Pharmaron has successfully developed a platform purification method for AAV gene therapies, which includes a highly adaptable toolbox for managing various AAV products and serotypes.
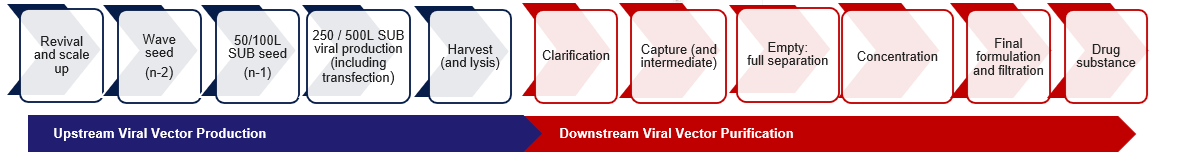
Image Credit: Pharmaron
Pharmaron’s manufacturing feasibility assessment, a small-scale, low-cost, and quick-turnaround examination of its scalable AAV manufacturing process, enables clients to determine whether the platform is a good fit for their product within six weeks.
Feasibility
The manufacturing feasibility evaluation provides information on product performance at the Ambr250 scale, using mini bioreactor technology, prior to small-scale downstream processing.
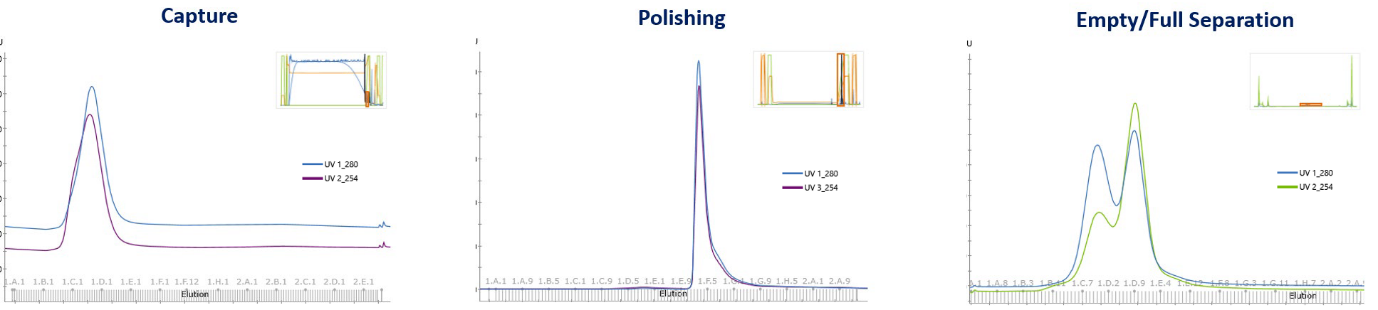
Image Credit: Pharmaron
Manufacturing feasibility delivers essential data on upstream reproducibility and downstream fit through small volume analytics, finding possibilities for process optimization, all while delivering quickly and lowering upfront costs.
HTPD optimization
Pharmaron’s Biomek i7® automated liquid handling system allows for the HTPD of gene therapy products, with DoE used to evaluate process parameters for optimization.
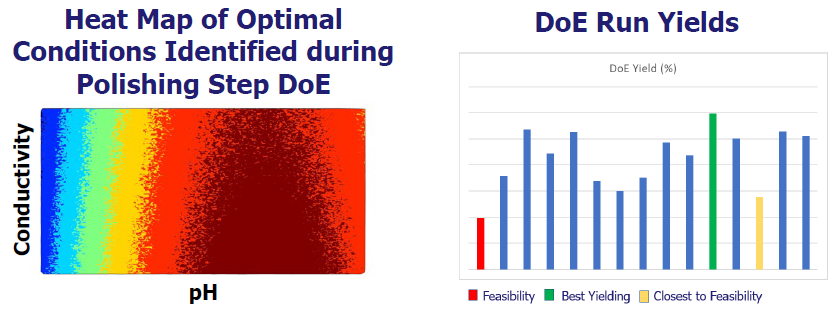
Image Credit: Pharmaron
|
|
DoE optimizes process conditions for the polishing stage, based on data from the manufacturing feasibility assessment.
|
Tiered testing
Sample prioritization is possible by integrating HTPD with Pharmaron’s tiered testing approach, resulting in lower costs and faster, data-driven decisions.
- HTP Analytical screening allows for rapid turnaround times, smaller sample volumes, and fewer assays, such as SEC titre and AEX-UPLC.
- Intermediate testing involves fewer samples, more tests, and higher accuracy, such as ddPCR, capsid ELISA, and Mass Photometry.
- Robustness and characterization mean a small fraction of specialized samples is required, such as DLS and AUC.
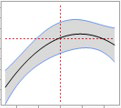
Rapid scale-up
Pharmaron’s platform provides constant scalability, from lab to GMP. Once optimization is complete, results are tested using lab-scale studies and demonstrated at pilot size, or technology is transported immediately into the GMP plant, depending on the partner's requirements.
Both the process and analytical data show consistency across scales. In the example below, the ideal circumstances mentioned above are duplicated at all scales.
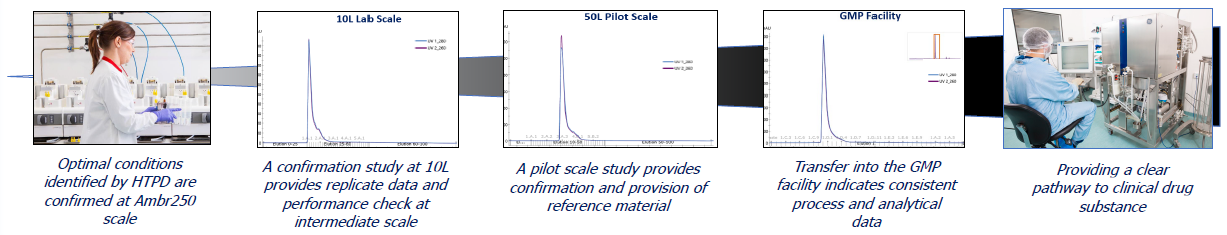
Image Credit: Pharmaron
Conclusion
- Pharmaron's platform method enables quick development from low-cost feasibility to a GMP-ready process in as little as six months.
- Pharmaron’s manufacturing feasibility assessment uses HTPD, design of experiment, and tiered testing to optimize unit operations.
- The platform's scalability allows for rapid confirmation, exemplification, and seamless technology transfer to Pharmaron’s GMP facility.
About Pharmaron
Pharmaron (Stock Code: 300759.SZ/3759.HK) is a premier R&D service provider for the life sciences industry. Founded in 2004, Pharmaron has invested in its people and facilities, and established a broad spectrum of research, development, and manufacturing service capabilities throughout the entire drug discovery, preclinical, and clinical development process across multiple therapeutic modalities, including small molecules, biologics, and CGT products. With over 17,000 employees and operations in China, the U.S., and the U.K., Pharmaron has an excellent track record in the delivery of R&D solutions to its partners in North America, Europe, Japan, and China.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.net, which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.
Last Updated: Jan 9, 2026